Quininib
- CAS NO.:143816-42-6
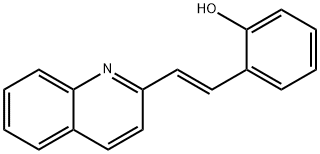
- Empirical Formula: C17H13NO
- Molecular Weight: 247.29
- MDL number: MFCD00186931
- Update Date: 2026-01-05 11:31:08
What is Quininib?
Description
Quininib is an antagonist of the cysteinyl leukotriene 1 (CysLT1) and CysLT2 receptors (IC50s = 1.4 and 52 μM, respectively). It inhibits tubule formation in HMEC-1 cells when used at concentrations of 3.16 and 10 μM and inhibits sprout formation in isolated mouse aortic rings in an ex vivo model of angiogenesis when used at 10 μM. Quininib (3 μM) decreases angiogenesis in an oxygen-induced retinopathy mouse model of ocular angiogenesis, preventing revascularization when used at concentrations of 0.5 and 3 μM, as well as increasing neovascularization and vascular density when used at 3 μM. It reduces tumor growth and inhibits angiogenesis in an HT-29 colorectal cancer mouse xenograft model when administered at a dose of 50 mg/kg every three days.
The Uses of Quininib
Quininib is a novel regulator of Ocular Angiogenesis, significantly inhibiting angiogenic tubule formation in HMEC-1 cells, angiogenic sprouting in aortic ring explants and retinal revascularisation in OIR mice.
Definition
ChEBI: Quininib is a styrylquinoline that is trans-2-styrylquinoline in which the the phenyl group has been substituted at position 2 by a hydroxy group. It is an anti-angiogenic compound that exerts a dose-dependent antagonism of the cysteinyl leukotriene pathway, preferentially antagonising cysteinyl leukotriene receptor 1. The major species at pH 7.3 It has a role as an angiogenesis inhibitor. It is a styrylquinoline and a member of phenols. It is functionally related to a trans-2-styrylquinoline. It is a conjugate base of a quininib(1+).
Properties of Quininib
| storage temp. | Store at -20°C |
| solubility | DMSO: soluble |
| form | A crystalline solid |
Safety information for Quininib
Computed Descriptors for Quininib
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
