Potassium tert-butoxide
Synonym(s):Potassium tert-butylate;Potassium tert-butylate;Potassium t-butoxide;Potassium-2-methylpropan-2-olate solution
- CAS NO.:865-47-4
- Empirical Formula: C4H9KO
- Molecular Weight: 112.21
- MDL number: MFCD00012162
- EINECS: 212-740-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:16
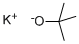
What is Potassium tert-butoxide ?
Description
Potassium t-butoxide (t-BuOK) is a strong, non-nucleophilic base. The pKa of its conjugate acid is about 17. Similar bases include sodium t-butoxide (t-BuONa) and lithium t-butoxide (t-BuOLi).
Chemical properties
white crystalline powder
The Uses of Potassium tert-butoxide
Potassium tert-Butoxide is used in the synthesis of many organic compounds primarily as a strong base. In particular, it is used as a reagent in the base- catalyzed carbonylation of amines for the synthesis of N-Formamides.
What are the applications of Application
To a suspension of methyl triphenylphosphonium bromide (2.4 g, 6.6 mmol) in THF was added tBuOK (1M in THF, 7.1 mmol) at RT. After stirring for 90 min, a solution of the SM (1.25 g, 3.35 mmol) in dry THF was added. The resulting mixture was stirred at RT for 18 h, after which time it was partitioned between saturated NH4Cl and MTBE. The org layer was dried (Na2SO4), concentrated, and purified by flash chromatography (25-30% EtOAc/hexane) to provide the product as a colorless oil. [0.3 g, 24%]
The Uses of Potassium tert-butoxide
Usually used for greener amidation of esters.
Synthesis
Potassium tert-butoxide is produced by reacting tert-butanol with potassium metal.
What are the applications of Application
Potassium tert-butoxide has been used as a strong base in the enantioselective synthesis of amines by transfer hydrogenation of N-(tertbutylsulfinyl)imines. It can also be used:
To synthesize aliphatic and aromatic amides from corresponding esters and amines.
As a base in the intramolecular cyclization of aryl ethers, amines, and amides.
As a catalyst to prepare styrene derivatives from aryl halides and alkenes by Mizoroki-Heck reaction.
Hazard
Potassium tert-butoxide is unstable and reactive; Reacts violently with water; Self-heating in large quantities, may catch fire; Causes burns; Inhalation may cause corrosive injuries to upper respiratory tract and lungs;
General Description
This product has been enhanced for catalytic efficiency.
Fire Hazard
Potassium tert-butoxide is a flammable solid.
It ignites on heating. Being very strongly
basic, its reactions with acids are highly
exothermic. Contact of solid powder with
drops of sulfuric acid and vapors of acetic
acid caused ignition after an induction period
of 0.5 and 3 minutes, respectively (Manwaring
1973). Ignition occurs upon reactions
with many common solvents of the
type ketone, lower alcohols, esters, and halogenated
hydrocarbons. Such solvents include
acetone, methyl ethyl ketone, methyl isobutyl
ketone, methanol, ethanol, n-propanol, isopropanol,
ethyl acetate, n-propyl formate,
n-butyl acetate, chloroform, methylene chloride,
carbon tetrachloride, epichlorohydrin,
dimethyl carbonate, and diethyl sulfate
(NFPA 1997). Such ignition may arise from
accidental contact of the alkoxide with these
solvents and may be attributed to sudden
release of energy from exothermic reactions.
However, slow mixing of the powder
with excess solvent will dissipate the
heat. It reacts violently with water, producing
tert-butanol and potassium hydroxide, as
follows:
K―OC―(C4H9)3+H2O →
tert-C4H9OH +KOH
The addition of potassium tert-butoxide
to the solvent dimethyl sulfoxide can cause
ignition of the latter (Bretherick 1995).
Flammability and Explosibility
Flammable
Purification Methods
It sublimes at 220o/1mm. The last traces of tert-BuOH are removed by heating at 150-160o/2mm for 1hour. It is best prepared afresh as likely impurities are tert-BuOH, KOH and K2CO3 depending on its exposure to air. Its solubility at 25-26o in hexane, toluene, Et2O, and THF is 0.27%, 2.27%, 4.34% and 25.0%, respectively. [Feuer et.al. J Am Chem Soc 78 4364, Doering & Urban J Am Chem Soc 78 5938 1956, Beilstein 1 IV 1612.]
Properties of Potassium tert-butoxide
| Melting point: | 256-258 °C (dec.) (lit.) |
| Boiling point: | 275°C |
| Density | 0.910 g/mL at 20 °C |
| vapor pressure | 1 mm Hg ( 220 °C) |
| Flash point: | 54 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in hexane, toluene, diethyl ether and terahydrofuran. |
| form | Solution |
| appearance | White solid |
| pka | pK1:18(25°C) |
| Specific Gravity | 0.902 |
| color | White to off-white |
| PH | 13 (5g/l, H2O, 20℃)Hydrolysis |
| Water Solubility | REACTS |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Sensitive | Moisture Sensitive |
| BRN | 3556712 |
| Stability: | Stable, but reacts violently with water and acids, possibly leading to fire. Incompatible with water, acids, halogenated hydrocarbons, alcohols, strong oxidizing agents, ketones, carbon dioxide. |
| CAS DataBase Reference | 865-47-4(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Methyl-2-propanol, potassium salt (865-47-4) |
Safety information for Potassium tert-butoxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H228:Flammable solids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium tert-butoxide
| InChIKey | LPNYRYFBWFDTMA-UHFFFAOYSA-N |
Potassium tert-butoxide manufacturer
Sainor Laboratories Pvt Ltd Unit III
SAKEM LLP
Zama Chemical
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Potassium tert butoxide 98%View Details
Potassium tert butoxide 98%View Details -
 Potassium tert-butoxide, 1M in THF 99%View Details
Potassium tert-butoxide, 1M in THF 99%View Details -
 Potassium tert-butoxide, 98% 99%View Details
Potassium tert-butoxide, 98% 99%View Details -
 Potassium tert-butylate CASView Details
Potassium tert-butylate CASView Details -
 Potassium Tert Butoxide, 98%, 100gm bottleView Details
Potassium Tert Butoxide, 98%, 100gm bottleView Details
865-47-4 -
 Potassium Tertiary Butoxide CAS No: 865-47-4View Details
Potassium Tertiary Butoxide CAS No: 865-47-4View Details
865-47-4 -
 Potassium Tert Butoxide, >99%, 100 KgView Details
Potassium Tert Butoxide, >99%, 100 KgView Details
865-47-4 -
 POTASSIUM TERT BUTOXIDE, >99%, 200 KGSView Details
POTASSIUM TERT BUTOXIDE, >99%, 200 KGSView Details
865-47-4
