PlatensiMycin
Synonym(s):Platensimycin, Streptomyces sp. - CAS 835876-32-9 - Calbiochem
- CAS NO.:835876-32-9
- Empirical Formula: C24H27NO7
- Molecular Weight: 441.474
- MDL number: MFCD14635440
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:24:39
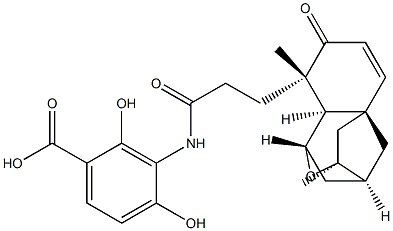
What is PlatensiMycin?
Description
Merck researchers isolated platensimycin, a natural antibiotic, from the bacterium?Streptomyces platensis?in 2006. The metabolite was later found to have potential for treating diabetes and related disorders. In 2011, B. Shen and co-workers deduced the details of platensimycin’s biosynthesis by?analyzing its biosynthetic gene clusters.
The Uses of PlatensiMycin
Platensimycin is a novel, broad spectrum, Gram-positive antibiotic produced by strains of Streptomyces platensis. Its discovery was heralded by high profile publication and commentary in the scientific and lay press. Platensimycin was discovered by a target-based, whole-cell screening strategy using an antisense differential sensitivity assay, based on the inhibition of fatty acid synthesis. Platensimycin inhibits bacterial growth by selectively inhibiting the elongation enzyme, b-ketoacyl acyl carrier protein synthase (FabF) of the fatty acid synthesis pathway.
The Uses of PlatensiMycin
Platensimycin is a broad spectrum gram-positive antibiotic.
What are the applications of Application
Platensimycin is a broad spectrum gram-positive antibiotic
Definition
ChEBI: A monocarboxylic acid amide obtained by the formal condensation of the amino group of 3-amino-2,4-dihydroxybenzoic acid with the carboxy group of the oxatetracyclic cage component. It is an antibiotic isolated from Streptomyces platensis a d exhibits inhibitory activity against fatty acid synthase.
Enzyme inhibitor
This thiol-reactive Streptomyces platensis-derived antibiotic (FW = 438.50 g/mol; CAS 835876-32-9), also named 3-[[3-[(1R,3R,4R,5aR,9R,9aS)- 1,4,5,8,9,9a-hexahydro-3,9-dimethyl-8-oxo-3H-1,4:3,5a-dimethano-2- benzoxepin-9-yl]-1-oxopropyl]amino]-2,4-dihydroxybenzoic acid, inhibits Staphylococcus aureus b-ketoacyl-[acyl-carrier-protein] synthase II, or FabF (IC50 = 290 nM). This enzyme, which allows bacteria to produce the fatty acids needed for making cell membranes, is thus a uniquely druggable prokaryotic target. Platensimycin has potent, broad-spectrum Gram-positive activity in vitro and exhibits no cross-resistance to other key antibiotic-resistant bacteria including Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate S. aureus, vancomycin-resistant Enterococci, as well as linezolid-resistant and macrolide-resistant pathogens. Platencin is a more potent analogue of Platensimycin. While effective in vivo when continuously administered, its efficacy is reduced when administered by more conventional means. Mechanism of Action: The Cys163 within the FabF active site is activated through the dipole moment of helix N-a-3, lowering its pKa, also increasing its nucleophilicity by the stabilizing effects of FabF’s catalytically required oxyanion hole. Interestingly, the crystal structure complex with platensimycin employed a C163Q mutant which gave a 50-fold increase in apparent binding.
Properties of PlatensiMycin
| Melting point: | 220-222 °C(Solv: nitromethane (75-52-5)) |
| Boiling point: | 683.1±55.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble1mg/mL, clear, colorless |
| form | Tan solid |
| pka | 2.14±0.13(Predicted) |
| color | white to beige |
Safety information for PlatensiMycin
Computed Descriptors for PlatensiMycin
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
