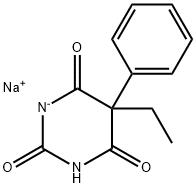
Phenobarbital sodium
Synonym(s):5-Ethyl-5-phenyl-2,4,6-trioxohexahydropyrimidine sodium salt;5-Ethyl-5-phenylbarbituric acid sodium salt;Luminal sodium salt;Sodium 5-ethyl-5-phenylbarbiturate
- CAS NO.:57-30-7
- Empirical Formula: C12H12N2O3.Na
- Molecular Weight: 254.22
- MDL number: MFCD00036211
- EINECS: 200-322-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:22:57
What is Phenobarbital sodium ?
Chemical properties
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2, Method II).
The Uses of Phenobarbital sodium
Phenobarbital sodium salt has been used:
- to anesthetize mice for inducing ischemia-reperfusion injury
- as a positive control for the activation of constitutive androstane receptor (CAR)
- in bioluminescent yeast bioreporters (BLYAS) assay
The Uses of Phenobarbital sodium
Anticonvulsant; sedative-hypnotic.
Definition
ChEBI: A barbiturate that is the sodium salt of phenobarbital (barbituric acid substituted at C-5 by ethyl and phenyl groups).
brand name
Luminal Sodium (Sterling Winthrop).
General Description
Odorless white crystalline powder. Aqueous solutions are alkaline to litmus and phenolphthalein (pH approximately 9.3). Bitter taste. A narcotic.
Air & Water Reactions
Hygroscopic. May be sensitive to prolonged exposure to air. Water soluble. Aqueous solutions are unstable, but may be kept at or below 50°F for a few days.
Reactivity Profile
Phenobarbital sodium can react with strong oxidizing agents. Incompatible with ammonium salts and chloral hydrate. Also incompatible with acids and acidic substances .
Fire Hazard
Flash point data for Phenobarbital sodium are not available; however, Phenobarbital sodium is probably combustible.
Biochem/physiol Actions
Phenobarbital, a substituted barbituric acid is an antileptic drug and is not easily eliminated from circulation. It hyperpolarizes the synaptic neuronal membranes by favoring the activation of neuronal postsynaptic GABAA?receptors by γ aminobutyric acid (GABA). Phenobarbital is also effective in treating neonatal seizures and status epilepticus.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Poison by ingestion, intravenous, intraperitoneal, intraduodenal, and subcutaneous routes. Human systemic effects by ingestion: nausea or vomiting and coma. Experimental reproductive effects. Mutation data reported. Used to treat epilepsy, as a hypnotic and sedative. When heated to decomposition it emits toxic fumes of NOx and NazO. See also BARBITURATES
Properties of Phenobarbital sodium
| Melting point: | 175°C |
| solubility | H2O: 1 g/mL |
| form | Solid |
| color | White to Off-White |
| PH | pH (100g/l, 25℃) : 9.0~11.0 |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| Merck | 13,7319 |
| BRN | 3802044 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 57-30-7(CAS DataBase Reference) |
| EPA Substance Registry System | Phenobarbital sodium (57-30-7) |
Safety information for Phenobarbital sodium
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Phenobarbital sodium
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran




You may like
-
 57-30-7 Phenobarbital sodium 98%View Details
57-30-7 Phenobarbital sodium 98%View Details
57-30-7 -
 57-30-7 98%View Details
57-30-7 98%View Details
57-30-7 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
