Abrocitinib
Synonym(s):N-[cis-3-(Methyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)cyclobutyl]-1-propanesulfonamide;N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}-propane-1-sulfonamide
- CAS NO.:1622902-68-4
- Empirical Formula: C14H21N5O2S
- Molecular Weight: 323.41
- MDL number: MFCD30187577
- Update Date: 2026-01-20 19:14:41
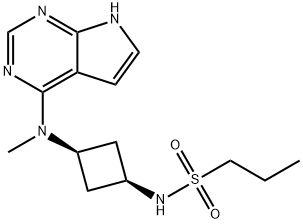
What is Abrocitinib?
Absorption
Abrocitinib is absorbed with over 91% extent of oral absorption and absolute oral bioavailability of approximately 60%. The peak plasma concentrations of abrocitinib are reached within one hour. Steady-state plasma concentrations of abrocitinib are achieved within 48 hours after once-daily administration. Both Cmax and AUC of abrocitinib increased dose proportionally up to 200 mg.
A high-fat meal, high-calorie meal increased AUC by 26% and Cmax by 29%, and prolongs Tmax by two hours; however, there are ultimately no clinically relevant effect on abrocitinib exposures.
Toxicity
There is no experience regarding human overdosage with abrocitinib. In clinical trials, there were no specific toxicities observed when abrocitinib was administered in single oral doses of 800 mg and 400 mg daily for 28 days. An overdose should be responded with symptomatic and supportive treatment, as there is no specific antidote for overdose with abrocitinib.
Characteristics
Class: non-receptor tyrosine kinase
Treatment: atopic dermatitis
Oral bioavailability = 60%
Elimination half-life = 5 h
Protein binding = 50%
Indications
Abrocitinib is indicated for the treatment of moderate-to-severe atopic dermatitis in adults who are candidates for systemic therapy. In the US, it is indicated to treat refractory, moderate-to-severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when the use of those therapies is inadvisable.
Abrocitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
Background
Abrocitinib is an oral small-molecule inhibitor of Janus kinase 1 (JAK1). Janus kinases are intracellular enzymes involved in transduction pathways that regulate hematopoiesis and immune cell function. The Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signalling pathway plays a central role in the pathogenesis of a variety of autoimmune and inflammatory diseases, including atopic dermatitis, a chronic inflammatory skin disease with complex pathogenesis. Atopic dermatitis is characterized by epidermal hyperplasia, skin barrier dysfunction, and the aberrant activation of immune cells. Patients with moderate-to-severe atopic dermatitis report reduced quality of life and often face limited treatment options. JAK inhibitors recently attracted more attention as potential treatments for inflammatory disorders, as JAK inhibition is associated with rapid and sustained anti-inflammatory efects.
Abrocitinib was approved by the European Commission on December 10, 2021, for the treatment of moderate-to-severe atopic dermatitis (AD) in adults who are candidates for systemic therapy. On January 14, 2022, the FDA approved abrocitinib for the treatment of refractory, moderate-to-severe AD in adults whose disease is not adequately controlled with other systemic drug products, including biologics, or when the use of those therapies is inadvisable. Health Canada also approved the use of abrocitinib in pediatric patients 12 years and older.
Biochem/physiol Actions
PF-04965842 is a Janus Kinase (JAK) inhibitor selective for JAK1 with an IC50 value of 29 nM for JAK1 compared to 803 nM for JAK2, >10000 nM for JAK3 and 1250 nM for Tyk2. JAKs mediate cytokine signaling, and are involved in cell proliferation and differentiation. PF-04965842 has been investigated as a possible treatment for psoriasis.
Mechanism of action
PF-04965842(Abrocitinib) is an orally bioavailable, selective JAK1 inhibitor to treat moderate-to-severe atopic dermatitis. Abrocitinib preferentially blocks cytokine signaling involving JAK1 and is selective against signaling pathways using dual JAK2 or JAK2/TYK2.
Pharmacokinetics
Abrocitinib mediates anti-inflammatory effects by blocking the signalling of pro-inflammatory cytokines implicated in atopic dermatitis. It dose-dependently reduces the serum markers of inflammation in atopic dermatitis, including high sensitivity C-reactive protein (hsCRP), interleukin-31 (IL-31), and thymus and activation regulated chemokine (TARC). These changes returned to near baseline within four weeks following drug discontinuation. At two weeks of treatment, the mean absolute lymphocyte count increased, which returned to baseline by nine months of treatment. Treatment with abrocitinib was associated with a dose-related increase in B cell counts and a dose-related decrease in NK cell counts: the clinical significance of these changes is unknown.
Treatment with 200 mg abrocitinib once-daily was associated with a transient, dose-dependent decrease in platelet count with the nadir occurring at a median of 24 days. Recovery of platelet count (~40% recovery by 12 weeks) occurred without discontinuation of the treatment.
Clinical Use
PF-04965842(Abrocitinib), a selective JAK1 inhibitor, was approved in 2022 for the treatment of adults with refractory, moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with other systemic drug products or when the use of those therapies is inadvisable.
Metabolism
Abrocitinib undergoes CYP-mediated oxidative metabolism. CYP2C19 is the predominant enzyme, accounting for about 53% of drug metabolism. CYP2C9 is responsible for 30% of drug metabolism. About 11% and 6% of the drug is metabolized by CYP3A4 and CYP2B6, respectively. In a human radiolabeled study, the parent drug was the most prevalent circulating species. Polar mono-hydroxylated metabolites of abrocitinib - M1 (3-hydroxypropyl; PF-06471658), M2 (2-hydroxypropyl; PF-07055087), and M4 (pyrrolidinone pyrimidine; PF-07054874) - were also identified in the systemic circulation. M2 has a chiral center, thus has an enantiomer M3 (PF-07055090). At steady state, M2 and M4 are major metabolites and M1 is a minor metabolite.
M2 has a pharmacological activity comparable to abrocitinib while M1 is less pharmacologically active than abrocitinib. M3 and M4 are inactive metabolites. The pharmacologic activity of abrocitinib is attributable to the unbound exposures of the parent molecule (~60%) as well as M1 (~10%) and M2 (~30%) in the systemic circulation. The sum of unbound exposures of abrocitinib, M1 and M2, each expressed in molar units and adjusted for relative potencies, is referred to as the abrocitinib active moiety.
Metabolism
The oral absorption of abrocotinib is 91%, but its oral bioavailability is moderate (60%) due to hepatic metabolism.3,4 Human PK studies with oral administration of [14C]-abrocitinib showed that the parent drug was the most abundant circulating species (26%), along with 3 oxidative metabolites: PF-06471658 (11%), PF-07055087 (12%), and PF- 07054874 (14%). The two hydroxyl compounds are active metabolites, with comparable JAK1 selectivity profiles to abrocitinib, whereas the pyrrolidinone pyrimidine metabolite lacks in kinase activity. Based on the in vitro cytochrome P450 phenotyping studies in human hepatocytes, CYP2C19 and CYP2C9 are the major CYP isoforms involved in the oxidative metabolism of abrocitinib, contributing to 53% and 30% of overall metabolism, respectively. As abrocotinib is primarily cleared in the liver, impairment in hepatocellular function may affect its pharmacokinetic properties, which could potentially impact its safety and/or efficacy. Abrocitinib is quite lipophilic with logD (pH 7.4) value of 1.9, which contributes to a modest plasma protein binding of 64% in humans. Its terminal half-life of 5 h along with 60% oral bioavailability appears to be adequate for once-daily, oral treatment of moderate-to-severe atopic dermatitis.
Properties of Abrocitinib
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO:100.0(Max Conc. mg/mL);309.2(Max Conc. mM) |
| form | powder |
| pka | 10.55±0.40(Predicted) |
| color | white to beige |
Safety information for Abrocitinib
Computed Descriptors for Abrocitinib
Abrocitinib manufacturer
LUPA PHARMACEUTICALS
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 1622902-68-4 Abrocitinib 98+View Details
1622902-68-4 Abrocitinib 98+View Details
1622902-68-4 -
 PF-04965842 CAS 1622902-68-4View Details
PF-04965842 CAS 1622902-68-4View Details
1622902-68-4 -
 Abrocitinib APIView Details
Abrocitinib APIView Details
1622902-68-4 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
