Pemoline
Synonym(s):2-Amino-5-phenyl4(5H)-oxazolone;Phenylisohydantoin
- CAS NO.:2152-34-3
- Empirical Formula: C9H8N2O2
- Molecular Weight: 176.17
- MDL number: MFCD00083197
- EINECS: 218-438-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
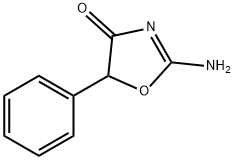
What is Pemoline?
Absorption
Pemoline is rapidly absorbed from the gastrointestinal tract
Toxicity
Side effects include insomnia, anorexia, stomach ache, skin rashes, increased irritability, mild depression, nausea, dizziness, headache, drowsiness, and hallucinations
Chemical properties
White Solid
Originator
Deltamine,Aron,France,1960
The Uses of Pemoline
A CNS stimulant. Controlled Substance
Indications
For treatment of Attention Deficit Hyperactivity Disorder (ADHD)
Background
In 2005, the Food and Drug Administration (FDA) withdrew approval for pemoline. In March 2005, Abbott Laboratories (Cylert marketer) had discontinued the production of Cylert arguing economic reasons.
Definition
ChEBI: A member of the class of 1,3-oxazoles that is 1,3-oxazol-4(5H)-one which is substituted by an amino group at position 2 and by a phenyl group at position 5. A central nervous system stimulant, it was used to treat hyperactivity disorders in children, but withdrawn from use following reports of serious hepatotoxicity.
Manufacturing Process
It is preferably prepared by reacting mandelic acid ethyl ester with guanidine in boiling alcoholic solution whereby it is obtained as difficultly soluble precipitate with a yield of 90%.
This compound is a white, crystalline compound melting at 256°-257°C with decomposition. It is readily soluble in concentrated aqueous alkali hydroxide solutions and in concentrated aqueous mineral acids.
brand name
Cylert (Abbott).
Therapeutic Function
Psychostimulant
World Health Organization (WHO)
Pemoline was introduced in 1975 for the treatment of attentiondeficit disorder. Because of its central stimulating effects it has also been used in weight control in combination with anorectic agents, laxatives.
General Description
Pemoline, 2-amino-5-phenyl-4(5H)-oxazolone (Cylert), hasa unique structure.
The compound is described as having an overall effect onthe CNS like that of methylphenidate. Pemoline requires 3to 4 weeks of administration, however, to take effect. A partialexplanation for the delayed effect may be that one of theactions of the agent, as observed in rats, is to increase therate of synthesis of DA.
Biological Activity
Long-acting central stimulant that induces self-injurious behavior in rats. Acts as an indirect monoamine agonist.
Pharmacokinetics
Pemoline belongs to the group of medicines called central nervous system (CNS) stimulants. It is used to treat attention deficit hyperactivity disorder (ADHD). Pemoline stimulates the brain, probably by affecting neurotransmitters, the chemicals in the brain that nerves use to communicate with each other.
Metabolism
Hepatic
Properties of Pemoline
| Melting point: | 255-256°C |
| Boiling point: | 307.77°C (rough estimate) |
| Density | 1.2662 (rough estimate) |
| refractive index | 1.5570 (estimate) |
| storage temp. | Store at RT |
| solubility | DMSO: soluble28mg/mL |
| form | solid |
| pka | pKa 10.5 (Uncertain) |
| color | white |
| CAS DataBase Reference | 2152-34-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 4(5H)-oxazolone, 2-amino-5-phenyl-(2152-34-3) |
Safety information for Pemoline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Pemoline
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran





You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
