ORANGE I
Synonym(s):α-Naphthol orange;4-(4-Hydroxy-1-naphthylazo)benzenesulfonic acid sodium salt;Orange I
- CAS NO.:523-44-4
- Empirical Formula: C16H11N2NaO4S
- Molecular Weight: 350.32
- MDL number: MFCD00021510
- EINECS: 208-346-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
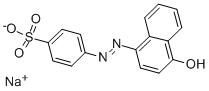
What is ORANGE I?
Chemical properties
Crystalline
The Uses of ORANGE I
A labeled form of alpha-Naphthol Orange, also known as Acid Orange 20, which is an organic azo dye.
Preparation
4-Aminobenzenesulfonic acid diazo, and Naphthalen-1-ol?coupling.
Definition
ChEBI: CI Acid Orange 20 is an organic molecular entity.
Purification Methods
Purify the dye by dissolving it in the minimum volume of H2O, adding, with stirring, a large excess of EtOH. The salt separates as orange needles. It is collected by centrifugation or filtration, washed with absolute EtOH (3x) and Et2O (2x) in the same way and dried in a vacuum desiccator over KOH. The free acid can be recrystallised from EtOH. [Slotta & Franke Chem Ber 64 86 1931, Beilstein 16 H 275, 16 II 117, 16 IV 410.] The purity can be checked by titration with titanium chloride [Klotz J Am Chem Soc 68 2299 1946].
Properties and Applications
orange. Soluble in water for palm light orange, slightly soluble in ethanol, acetone, insoluble in most organic solvents. The strong sulfuric acid for purple, diluted into palm orange, red with purple precipitation. Its water solution to join strong hydrochloric acid for orange brown; Add sodium hydroxide in red. Used for wool, silk fiber dyeing, etc. Also used in leather dyeing and indicator.
Properties of ORANGE I
| Melting point: | 260 °C (decomp) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| Colour Index | 14600 |
| color | Red to Very Dark Red |
| Merck | 14,6857 |
| BRN | 3826844 |
| IARC | 3 (Vol. 8, Sup 7) 1987 |
| EPA Substance Registry System | C.I. Acid Orange 20 monosodium salt (523-44-4) |
Safety information for ORANGE I
Computed Descriptors for ORANGE I
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

![4-[4-[[2-methyl-4-[[(p-tolyl)sulphonyl]oxy]phenyl]azo]anilino]-3-nitrobenzenesulphonic acid](https://img.chemicalbook.in/CAS/GIF/52636-61-0.gif)




You may like
-
 Acid orange 20 523-44-4 98%View Details
Acid orange 20 523-44-4 98%View Details
523-44-4 -
 Naphthol orange CAS 523-44-4View Details
Naphthol orange CAS 523-44-4View Details
523-44-4 -
 α-Naphthol Orange CAS 523-44-4View Details
α-Naphthol Orange CAS 523-44-4View Details
523-44-4 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
