OMadacycline (tosylate)
- CAS NO.:1075240-43-5
- Empirical Formula: C36H48N4O10S
- Molecular Weight: 728.86
- MDL number: MFCD28167752
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:17:46
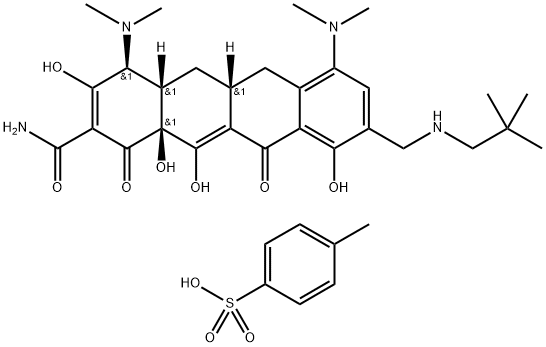
What is OMadacycline (tosylate)?
Description
Omadacycline tosylate is a semisynthetic tetracycline-class antibacterial agent, which has been designated by some as a “new-generation tetracycline” or “the first in a new class of aminomethylcyclines.” Omadacycline has been structurally modified to provide effectiveness against certain strains of bacteria that have mechanisms to resist the action of older tetracyclines. The new agent binds to the 30S ribosomal unit, blocks bacterial protein synthesis, and is generally bacteriostatic[1]. Omadacycline tosylate has broad-spectrum antibacterial activity against aerobic and anaerobic Gram-positive and Gram-negative bacteria, as well as atypical bacteria. Omadacycline tosylate has been studied in acute bacterial skin and skin structure infections, community-acquired pneumonia and urinary tract infections.
The Uses of OMadacycline (tosylate)
OMadacycline (tosylate) is a new type of 9-aminomethylcycline drug, which is a semi-synthetic compound obtained by modifying chemical groups on the basis of minocycline. The modification can help omacycline overcome bacterial resistance. Pharmacological properties, expand the antibacterial spectrum, and improve the pharmacokinetic properties.
Biological Activity
OMadacycline (tosylate) is an aminecycline antibiotic with antibacterial activity against Gram-positive and negative aerobic bacteria and some anaerobic bacteria.
Mode of action
(1) OMadacycline (tosylate) specifically binds to the A site of the 30S subunit of the bacterial ribosome, inhibits the normal binding of aminoacyl-tRNA to this site, terminates the elongation of the peptide chain, and blocks the synthesis of proteins to produce a bacteriostatic effect.
(2) The electron-donating group (dimethylamino) of the modified group of it can improve the activity and help to overcome the bacterial efflux pump resistance mechanism.
(3) The aminomethyl modification of it can make it overcome the bacterial ribosome protection mechanism (caused by the ribosome protection protein gene TetO) and enhance oral bioavailability.
(4) But it cannot resist the effect of Tet(X) modification enzyme.
References
[1] DANIEL A. HUSSAR; Elias B C. Omadacycline tosylate, Sarecycline hydrochloride, Rifamycin sodium, and Moxidectin[J]. Journal of the American Pharmacists Association, 2019. DOI:10.1016/j.japh.2019.07.016.
Properties of OMadacycline (tosylate)
| storage temp. | 4°C, protect from light |
| solubility | Soluble in DMSO |
| form | Solid |
| color | White to yellow |
Safety information for OMadacycline (tosylate)
Computed Descriptors for OMadacycline (tosylate)
| InChIKey | SETFNHZTVGTBHC-MPRIKUDSNA-N |
| SMILES | S(C1C=CC(C)=CC=1)(O)(=O)=O.O[C@@]12C(C(C(=O)N)=C(O)[C@@H](N(C)C)[C@]1([H])C[C@]1([H])CC3=C(C=C(CNCC(C)(C)C)C(O)=C3C(=O)C1=C2O)N(C)C)=O |&1:12,20,24,27,r| |
OMadacycline (tosylate) manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 Omadacycline Tosylate 97%View Details
Omadacycline Tosylate 97%View Details -
 1075240-43-5 98%View Details
1075240-43-5 98%View Details
1075240-43-5 -
 Omadacycline tosylate CAS 1075240-43-5View Details
Omadacycline tosylate CAS 1075240-43-5View Details
1075240-43-5 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
