Olodaterol
- CAS NO.:868049-49-4
- Empirical Formula: C21H26N2O5
- Molecular Weight: 386.44
- MDL number: MFCD16619349
- EINECS: 813-858-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-12 17:28:22
What is Olodaterol?
Absorption
Olodaterol reaches maximum plasma concentrations generally within 10 to 20 minutes following drug inhalation. In healthy volunteers, the absolute bioavailability of olodaterol following inhalation was estimated to be approximately 30%, whereas the absolute bioavailability was below 1% when given as an oral solution. Thus, the systemic availability of olodaterol after inhalation is mainly determined by lung absorption, while any swallowed portion of the dose only negligibly contributes to systemic exposure.
Toxicity
Adverse drug reactions that occurred at a frequency greater than 2% include nasopharyngitis (11.3%), upper respiratory tract infection (8.2%), bronchitis (4.7%), urinary tract infection (2.5%), cough (4.2%), dizziness (2.3%), rash (2.2%), diarrhea (2.9%), back pain (3.5%), and arthralgia (2.1%).
Description
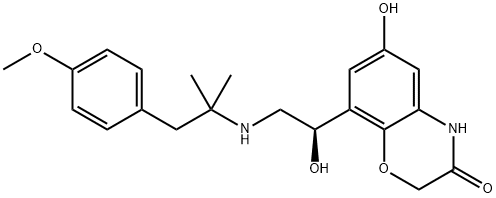
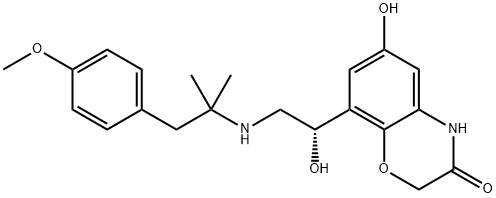
In 2013, olodaterol (also known BI-1744 CL) was approved for the treatment of chronic obstructive pulmonary disease (COPD) in Russia (March), Canada (June), and the EuropeanUnion (October). Olodaterol is delivered via the Respimat? Soft Mist? inhaler. Olodaterol was discovered from an effort to identify a β2-adrenergic receptor agonist that could be given once daily and with a superior safety profile over known β2- adrenergic receptor agonists. Olodaterol is a potent β2 agonist (EC50=0.1 nM, intrinsic activity=88%) and is highly selective for the β2 receptor over β1 and β3 receptors. In preclinical models in guinea pigs and dogs, olodaterol had a rapid onset of action and provided bronchoprotection over 24 h. Mechanistic studies indicated that olodaterol forms a highly stable complex with the β2-adrenergic receptor, with a dissociation half-life of 17.8 h. The synthesis of olodaterol proceeds through an (R)-styrene epoxide that is prepared via enantioselective reduction of an α-chloroketone intermediate. Ring opening of the epoxide with 1-(4- methoxyphenyl)-2-methylpropan-2-amine provides olodaterol.
Originator
Boehringer-Ingelheim (European Union)
The Uses of Olodaterol
Olodaterol-d3 is labelled Olodaterol (O262000) which is a long acting b-adrenoceptor agonist used as an inhalation for treating patients with chronic obstructive pulmonary disease (COPD).
Background
Olodaterol is a novel, long-acting beta2-adrenergic agonist (LABA) that exerts its pharmacological effect by binding and activating beta2-adrenergic receptors located primarily in the lungs. Beta2-adrenergic receptors are membrane-bound receptors that are normally activated by endogenous epinephrine whose signalling, via a downstream L-type calcium channel interaction, mediates smooth muscle relaxation and bronchodilation. Activation of the receptor stimulates an associated G protein which then activates adenylate cyclase, catalyzing the formation of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA). Elevation of these two molecules induces bronchodilation by relaxation of airway smooth muscles. It is by this mechanism that olodaterol is used for the treatment of chronic obstructive pulmonary disease (COPD) and the progressive airflow obstruction that is characteristic of it. Treatment with bronchodilators helps to mitigate associated symptoms such as shortness of breath, cough, and sputum production. Single doses of olodaterol have been shown to improve forced expiratory volume in 1 sec (FEV1) for 24 h in patients with COPD, allowing once daily dosing. A once-a-day treatment with a LABA has several advantages over short-acting bronchodilators and twice-daily LABAs including improved convenience and compliance and improved airflow over a 24-hour period. Despite similarities in symptoms, olodaterol is not indicated for the treatment of acute exacerbations of COPD or for the treatment of asthma.
Indications
Olodaterol is indicated for use in chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. It is not indicated for the treatment of acute exacerbations of COPD or for the treatment of asthma.
Definition
ChEBI: A member of the class of benzoxazine that is 6-hydroxy-1,4-benzoxazin-3-one in which the hydrogen at position 4 is replaced by a (1R)-1-hydroxy-2-{[1-(4-methoxyphenyl)-2-methylpropan-2-yl]amino}ethyl group. Used (as its hydrochloride salt) for long-term treatment of airflow obstruction in patients with chronic obstructive pulmonary disease including chronic bronchitis and/or emphysema.
brand name
Striverdi
Pharmacokinetics
Olodaterol is a potent agonist of the human beta2-adrenergic receptor in vitro, and is highly selective for this receptor, with much lower levels of activity at the b1- and b3-adrenergic receptors that are commonly expressed on cardiac smooth muscle and adipose tissue, respectively. Binding to the receptor causes smooth muscle relaxation in the lungs and bronchodilation. It has also been shown to potently reverse active bronchoconstriction.
Metabolism
Olodaterol is substantially metabolized by direct glucuronidation and by O-demethylation at the methoxy moiety followed by conjugation. Of the six metabolites identified, only the unconjugated demethylation product binds to beta2-receptors. This metabolite, however, is not detectable in plasma after chronic inhalation of the recommended therapeutic dose. Cytochrome P450 isozymes CYP2C9 and CYP2C8, with negligible contribution of CYP3A4, are involved in the O-demethylation of olodaterol, while uridine diphosphate glycosyl transferase isoforms UGT2B7, UGT1A1, 1A7, and 1A9 were shown to be involved in the formation of olodaterol glucuronides.
Properties of Olodaterol
| Boiling point: | 649.0±55.0 °C(Predicted) |
| Density | 1.250 |
| solubility | DMF: 20 mg/ml; DMF:PBS (pH 7.2) (1:3): 0.25 mg/ml; DMSO: 15 mg/ml; Ethanol: 1 mg/ml |
| form | A crystalline solid |
| pka | 9.42±0.20(Predicted) |
| color | Light yellow to khaki |
| Stability: | Hygroscopic |
Safety information for Olodaterol
Computed Descriptors for Olodaterol
Olodaterol manufacturer
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
![Methyl 2-oxo-3-((2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl)Methyl)-2,3-dihydro-1H-benzo[d]iMidazole-4-carboxylate](https://img.chemicalbook.in/CAS/20130318/GIF/CB02611251.gif)
![1-[[2'-(AMinoiMinoMethyl)[1,1'-biphenyl]-4-yl]Methyl]-2-ethoxy-1H-benziMidazole-7-carboxylic Acid](https://img.chemicalbook.in/CAS/20150408/GIF/1442400-65-8.gif)
![(Z)-Methyl 3-((2'-(N'-hydroxycarbaMiMidoyl)biphenyl-4-yl)Methyl)-2-oxo-2,3-dihydro-1H-benzo[d]iMidazole-4-carboxylate](https://img.chemicalbook.in/CAS/20130318/GIF/CB12611218.gif)
![1H-BenziMidazole-7-carboxylic acid, 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl]Methyl] -2-ethoxy-, ethyl ester](https://img.chemicalbook.in/CAS/GIF/1403474-70-3.gif)
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
