olivetolic acid
- CAS NO.:491-72-5
- Empirical Formula: C12H16O4
- Molecular Weight: 224.25
- MDL number: MFCD28383644
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-27 08:46:37
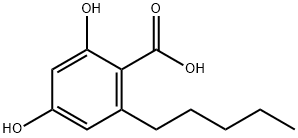
What is olivetolic acid?
The Uses of olivetolic acid
Olivetolic Acid is a precursor in the synthesis of primin and tetrahydrocannabinol.
What are the applications of Application
Olivetolic Acid is a key metabolic intermediate in the biosynthesis of cannabinoids
Definition
ChEBI: A member of the class of benzoic acids that is salicylic acid in which the hydrogens ortho- and para- to the carboxy group are replaced by a pentyl and a hydroxy group, respectively.
Biosynthesis
Olivetolic acid (OLA) biosynthesis involves the condensation of one hexanoyl-CoA with three malonyl-CoAs by a type III PKS (tetraketide synthase) OLA synthase (C. sativa OLS; CsOLS) and an OLA cyclase (CsOAC), both enzymes were derived from the Cannabis species (C. sativa). Studies have achieved trace amount of OLA production in both E. coli and S. cerevisia[1]. Ma et al. find that Pseudomonas sp LvaE encoding a short-chain acyl-CoA synthetase can efficiently convert hexanoic acid to hexanoyl-CoA. The co-expression of the acetyl-CoA carboxylase, the pyruvate dehydrogenase bypass, the NADPH-generating malic enzyme, as well as the activation of peroxisomal β-oxidation pathway and ATP export pathway are effective strategies to redirect carbon flux toward OLA synthesis.
References
[1] Ma, J, Y. Gu, and P. Xu . "Biosynthesis of cannabinoid precursor olivetolic acid by overcoming rate-limiting steps in genetically engineered Yarrowia lipolytica." Cold Spring Harbor Laboratory(2021).
Properties of olivetolic acid
| Melting point: | 143 °C |
| Boiling point: | 403.9±25.0 °C(Predicted) |
| Density | 1.237±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 3.41±0.34(Predicted) |
| color | Light Beige to Light Brown |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C12H16O4/c1-2-3-4-5-8-6-9(13)7-10(14)11(8)12(15)16/h6-7,13-14H,2-5H2,1H3,(H,15,16) |
Safety information for olivetolic acid
Computed Descriptors for olivetolic acid
| InChIKey | SXFKFRRXJUJGSS-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C1=C(CCCCC)C=C(O)C=C1O |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 491-72-5 Olivetolic acid 98%View Details
491-72-5 Olivetolic acid 98%View Details
491-72-5 -
 Olivetolic Acid Cas 491-72-5View Details
Olivetolic Acid Cas 491-72-5View Details
491-72-5 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
