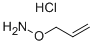
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
Synonym(s):O-(2-Propenyl)hydroxylamine hydrochloride
- CAS NO.:38945-21-0
- Empirical Formula: C3H8ClNO
- Molecular Weight: 109.55
- MDL number: MFCD00012957
- EINECS: 254-203-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:12:00
What is O-ALLYLHYDROXYLAMINE HYDROCHLORIDE?
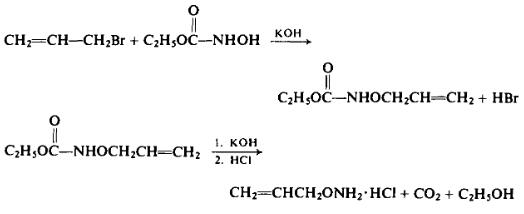
Preparation
To a solution of 86.8 gm (1.55 moles) of potassium hydroxide in 330 ml of absolute ethanol is added a solution of 159 gm (1.52 moles) of ethyl N-hydroxycarbamate in 330 ml of absolute ethanol. With external cooling to maintain an internal temperature of 25°C to this mixture is added 195.5 gm (1.62 moles) of allyl bromide. After the addition has been completed, the mixture is heated under reflux for 2 hr. After separating the potassium bromide formed during the reaction and washing it with absolute alcohol, the alcoholic solution is evaporated under reduced pressure. The residue is dissolved in ether and then the ether solution is extracted repeatedly with 10% aqueous sodium hydroxide solution. [From the ether solution, on evaporation 25.5 gm (18%) of ethyl N,O-diallylhydroxycar-bamate, b.p. 91-92°C (8.55 mm Hg) may be isolated.] The aqueous extract is acidified with 10% aqueous sulfuric acid and the ethyl O-allylhy-droxycarbamate is extracted with ether. Upon evaporating the ether off, 134.2 gm (61%) of the intermediate product, b.p. 107°C (12.5 mm Hg), is isolated.
In a steam distillation apparatus, 134 gm of ethyl O-allylhydroxy-carbamate is treated with a solution of 120 gm of potassium hydroxide in 280 ml of water. The product is steam-distilled into a receiver containing dilute hydrochloric acid.
The steam distillate is evaporated under reduced pressure. The residue is taken up twice in absolute ethanol and dried by evaporation under reduced pressure. The yield is 91 gm (55%, overall), m.p. 169-170°C. Upon recrystallization from absolute alcohol and dry ether, the melting point is raised to 170.6-170.8°C (172-174°C). Free O-allylhydrox-ylamine has the following reported properties: b.p. 98-99°C, n25D 1.4300.
The problems connected with the preparation of O-arylhydroxylamines by this method have been attributed to the instability of the aryl- substituted aminooxy group to the hydrolytic system used in its preparation. This problem has recently been circumvented by substituting for ethyl N-hydroxycarbamate, t-butyl N-hydroxycarbamate.
Properties of O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
| Melting point: | ~170 °C (dec.) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | powder to crystal |
| color | White to Almost white |
| BRN | 3552394 |
Safety information for O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
| InChIKey | XIQUJVRFXPBMHS-UHFFFAOYSA-N |
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
![5-CHLORO-4-(3-([(2-FURYLMETHYLENE)AMINO]OXY)-3-OXOPROP-1-ENYL)-1,3-DIMETHYL-1H-PYRAZOLE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6684886.gif)
![2-(5-METHYLISOXAZOL-3-YL)-5-(2-([(2,3,3-TRICHLOROALLANOYL)OXY]IMINO)PROPYL)-2H-1,2,3,4-TETRAAZOLE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6736147.gif)
![6-(2-([(3-CHLORO-2-PROPENYL)OXY]IMINO)ETHYL)-1-(3,4-DIMETHOXYPHENETHYL)-4-OXO-1,4-DIHYDRO-5-PYRIMIDINECARBONITRILE](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB6385992.gif)
![N-[BIS(4-FLUOROPHENYL)METHYLENE]-N-[(2,3,3-TRICHLOROALLANOYL)OXY]AMINE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB8102665.gif)


![4-[3-CHLORO-5-(TRIFLUOROMETHYL)-2-PYRIDINYL]-2-(3-[4-(DIMETHYLAMINO)PHENYL]-2-PROPENYLIDENE)-5-OXO-2,5-DIHYDROISOXAZOL-2-IUM-3-OLATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB0205836.gif)
![O1-[3-(5-CHLORO-1,3-DIMETHYL-1H-PYRAZOL-4-YL)ACRYLOYL]-4-(TERT-BUTYL)BENZENE-1-CARBOHYDROXIMAMIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB9167115.gif)
You may like
-
 O-Allylhydroxylamine hydrochloride 98% CAS 38945-21-0View Details
O-Allylhydroxylamine hydrochloride 98% CAS 38945-21-0View Details
38945-21-0 -
 O-Allylhydroxylamine hydrochloride 98.00% CAS 38945-21-0View Details
O-Allylhydroxylamine hydrochloride 98.00% CAS 38945-21-0View Details
38945-21-0 -
 O-Allylhydroxylamine Hydrochloride CAS 38945-21-0View Details
O-Allylhydroxylamine Hydrochloride CAS 38945-21-0View Details
38945-21-0 -
 O-Allylhydroxylamine hydrochloride CAS 38945-21-0View Details
O-Allylhydroxylamine hydrochloride CAS 38945-21-0View Details
38945-21-0 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
