Nickel(II) acetylacetonate
Synonym(s):2,4-Pentanedione nickel(II) derivative;Bis(acetylacetonato)nickel(II), Bis(2,4-pentanedionato) nickel(II);Ni(acac)2;Nickel(II) acetylacetonate
- CAS NO.:3264-82-2
- Empirical Formula: C10H14NiO4
- Molecular Weight: 256.91
- MDL number: MFCD00000024
- EINECS: 221-875-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:53
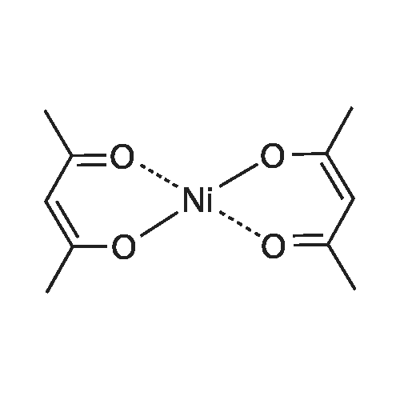
What is Nickel(II) acetylacetonate?
Chemical properties
Nickel(II) acetylacetonate Ni(CH3COCHCOCH3)2, is obtained as emerald green crystals by dehydration of the dihydrate at 50°C in vacuum; it has an unusual trimeric structure in the solid state. The dihydrate Ni(CH3COCH3)2.2H2O is prepared by the addition of acetylacetone to solutions of nickel(II) salts in the presence of a weak base such as sodium acetate. Nickel(II) acetylacetonate is soluble in organic solvents and finds use in the synthesis of organometallic compounds such as nickelocene and bis(cyclooctadiene) nickel. It is also industrially important as a catalyst component in the oligomerization of alkenes and in the conversion of acetylene to cyclooctatetraene.
The Uses of Nickel(II) acetylacetonate
Nickel(II) acetylacetonate is usefully soluble in organic solvents and is therefore used in the Grignard synthesis of nickelocene from cyclopentadienyl magnesium halides. More ionic nickel(II) salts such as the thiocyanate can be reacted with potassium cyclopentadienide in liquid ammonia.
The Uses of Nickel(II) acetylacetonate
Catalyst for organic reactions.
The Uses of Nickel(II) acetylacetonate
Nickel Acetylacetonate is used in preparation method of Bisphenol Dipropargyl Ether and Cyanate Ester blending resin.
Preparation
Nickel(II) acetylacetonate is isolated as the dihydrate Ni(acac)2·2H2O from aqueous solutions of nickel(II) salts in the presence of acetylacetone and a weak base such as sodium acetate; it is readily dehydrated to the green anhydrous compound at 50° in vacuo. From ammoniacal solutions of nickel(II) salts the diammoniate Ni(acac)2·2NH3 precipitates; this also readily evolves the two extra ligands to give the green acetylacetonate.
Flammability and Explosibility
Not classified
Safety Profile
Confirmed human carcinogen. Poison by intraperitoneal route. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Wash the green solid with H2O, dry it in a vacuum desiccator and recrystallise it from MeOH. [Charles & Pawlikowski J Phys Chem 62 440 1958.] The complex can be conveniently dehydrated by azeotropic distillation with toluene, and the crystals may be isolated by concentrating the toluene solution. [Wilkinson et al. J Am Chem Soc 76 1970 1954, Beilstein 1 IV 3677.]
Properties of Nickel(II) acetylacetonate
| Melting point: | 230 °C (dec.)(lit.) |
| Boiling point: | 220 °C (11 mmHg) |
| Density | 0,145 g/cm3 |
| vapor pressure | 2.7 hPa (110 °C) |
| refractive index | 1.57-1.64 |
| Flash point: | >200°C |
| storage temp. | Store below +30°C. |
| solubility | 4.8g/l |
| form | Liquid |
| color | Clear pale yellow |
| Specific Gravity | 1.455 |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,6500 |
| BRN | 4157970 |
| Exposure limits | NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 3264-82-2 |
| NIST Chemistry Reference | Nickel acetylacetonate(3264-82-2) |
| EPA Substance Registry System | Nickel, bis(2,4-pentanedionato-.kappa.O,.kappa.O')-, (SP-4-1)- (3264-82-2) |
Safety information for Nickel(II) acetylacetonate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Nickel(II) acetylacetonate
| InChIKey | YAGMVFMEBGIOTO-FGSKAQBVSA-M |
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
3264-82-2 -
 Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
3264-82-2 -
 Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
3264-82-2 -
 Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
Nickel(II) 2,4-pentanedionate CAS 3264-82-2View Details
3264-82-2 -
 Nickel(II) acetylacetonate, 95% CAS 3264-82-2View Details
Nickel(II) acetylacetonate, 95% CAS 3264-82-2View Details
3264-82-2 -
 Nickel acetylacetonate 98.00% CAS 3264-82-2View Details
Nickel acetylacetonate 98.00% CAS 3264-82-2View Details
3264-82-2 -
 Nickel acetylacetonate 98% CAS 3264-82-2View Details
Nickel acetylacetonate 98% CAS 3264-82-2View Details
3264-82-2 -
 Nickel(II) acetylacetonate CAS 3264-82-2View Details
Nickel(II) acetylacetonate CAS 3264-82-2View Details
3264-82-2
