Methohexital
- CAS NO.:151-83-7
- Empirical Formula: C14H18N2O3
- Molecular Weight: 262.3
- MDL number: MFCD00864449
- EINECS: 205-798-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:17
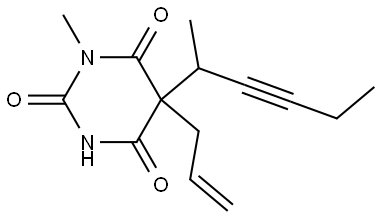
What is Methohexital?
Absorption
The absolute bioavailability following rectal administration of methohexital is 17%.
Toxicity
The onset of toxicity following an overdose of intravenously administered methohexital will be within seconds of the infusion. If methohexital is administered rectally or is ingested, the onset of toxicity may be delayed. The manifestations of an ultrashort-acting barbiturate in overdose include central nervous system depression, respiratory depression, hypotension, loss of peripheral vascular resistance, and muscular hyperactivity ranging from twitching to convulsive-like movements. Other findings may include convulsions and allergic reactions. Following massive exposure to any barbiturate, pulmonary edema, circulatory collapse with loss of peripheral vascular tone, and cardiac arrest may occur.
The Uses of Methohexital
Methohexital is a short-acting barbiturate used as a sedative. Methohexital is used an anesthetic for oral surgery and dentistry and is also used to induce anesthesia prior to electroconvulsive therapy (ECT). Methohexital is a Controlled Substance.
Indications
Methohexital is indicated for use as an intravenous anaesthetic. It has also been commonly used to induce deep sedation.
Background
An intravenous anesthetic with a short duration of action that may be used for induction of anesthesia.
Definition
ChEBI: A barbiturate, the structure of which is that of barbituric acid substituted at N-1 by a methyl group and at C-5 by allyl and 1-methylpent-2-ynyl groups.
Pharmacokinetics
Methohexital, a barbiturate, is used for the induction of anesthesia prior to the use of other general anesthetic agents and for induction of anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli. Little analgesia is conferred by barbiturates; their use in the presence of pain may result in excitation.
Metabolism
Metabolism occurs in the liver through demethylation and oxidation. Side-chain oxidation is the most important biotransformation involved in termination of biologic activity.
Properties of Methohexital
| Melting point: | 96 °C(Solv: ethanol (64-17-5)) |
| Density | 1.113 |
| storage temp. | 2-8°C |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| pka | 7.82±0.10(Predicted) |
| form | Solid |
| color | White to Off-White |
| NIST Chemistry Reference | Methohexitone(151-83-7) |
Safety information for Methohexital
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Methohexital
Methohexital manufacturer
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran




You may like
-
 151-83-7 / 18652-93-2 Methohexital 98%View Details
151-83-7 / 18652-93-2 Methohexital 98%View Details
151-83-7 / 18652-93-2 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
