MAGNESIUM SULFIDE
- CAS NO.:12032-36-9
- Empirical Formula: MgS
- Molecular Weight: 56.37
- MDL number: MFCD00016207
- EINECS: 234-771-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-31 14:38:37
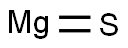
What is MAGNESIUM SULFIDE?
Chemical properties
Red-brown, crystalline solid.decomposes in water.
Chemical properties
Magnesium Sulfide is a red-brown, crystalline solid, decomposes above 2000 C, decomposes in water.
Physical properties
MgS is a white crystalline material but is often
encountered in an impure form that is a brown noncrystalline
powder.
The chemical properties of MgS resemble those of
related ionic sulfides such as those of Na, Ba, and Ca.
Magnesium Sulfide reacts with oxygen to form the corresponding sulfate,
magnesium sulfate. MgS reacts with water to give
hydrogen sulfide and magnesium hydroxide.
The Uses of MAGNESIUM SULFIDE
Magnesium Sulfide is the source of hydrogen sulfide, laboratory reagent.
Preparation
Magnesium Sulfide
can be prepared by the direct reaction of the elements,
calcined in an inert atmosphere, at a 1:1.1 molecular
ratio:
Mg+ S+ heat→MgS
MgS also forms by the reaction of hydrogen sulfide gas with soluble magnesium salts in solution.
Aside from being a component
of some slags, MgS is a rare nonterrestial mineral
“niningerite” detected in some meteorites.
Magnesium sulfide is available commercially from
a number of suppliers.
Properties of MAGNESIUM SULFIDE
| Melting point: | >2000°C (dec.) |
| Density | 2,84 g/cm3 |
| solubility | reacts with H2O |
| form | red-brown cubic crystals |
| color | red-brown cubic crystals, crystalline |
| Water Solubility | decomposes in H2O [HAW93] |
| EPA Substance Registry System | Magnesium sulfide (MgS) (12032-36-9) |
Safety information for MAGNESIUM SULFIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for MAGNESIUM SULFIDE
MAGNESIUM SULFIDE manufacturer
New Products
Cyclopropane-1,1-dicarboxylic acid Cyclopentane-1,2-dione 2-Bromo-5-iodopyridine 4-isothiocyanato-2-(trifluoroMethyl)benzonitrile Amino-4-methoxyben-zeneacetic acid 3-Aminophenylacetic acid 1-AMino-cyclobutaneMethanol HCl (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Cefuroxime EP Impurity-A N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 4-Bromo Benzylcyanide 5-azidovalericacid 4-bromo-1H-pyrazole-3-carboxylic acid Isopropyl amine Hydrochloride tert-butyl 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-vinyl-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylateRelated products of tetrahydrofuran

You may like
-
 Magnesium sulphide CAS 12032-36-9View Details
Magnesium sulphide CAS 12032-36-9View Details
12032-36-9 -
 Magnesium SulphideView Details
Magnesium SulphideView Details
12032-36-9 -
 Magnesium Sulphide sView Details
Magnesium Sulphide sView Details
12032-36-9 -
 4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
6939-69-1 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8
