LUMIRACOXIB
- CAS NO.:220991-20-8
- Empirical Formula: C15H13ClFNO2
- Molecular Weight: 293.72
- MDL number: MFCD07186254
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
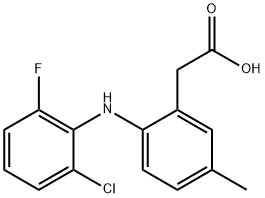
What is LUMIRACOXIB?
Absorption
Rapidly absorbed following oral administration, with an absolute oral bioavailablity of 74%.
Toxicity
Single oral doses in mice and rats resulted in mortality and/or moribundity at doses of 600 mg/kg and 500 mg/kg, respectively. Single intraperitoneal doses in mice and rats results in mortality/moribundity at 750 mg/kg and 1000 mg/kg, respectively. The maximum non-lethal single oral and intraperitoneal dose in mouse was 300 mg/kg and 250 mg/kg, respectively. In the rat it was 150 mg/kg and 250 mg/kg, respectively.
Description
As a second-generation, selective cyclooxygenase (COX-2) inhibitor, lumiracoxib is devoid of the gastrointestinal issues that plague other non-selective, nonsteroidal, anti-inflammatory drugs (NSAIDs) that crossover to COX-1. As an inhibitor of the inducible COX-2 that is up-regulated in pathological processes of pain and inflammation, lumiracoxib blocks the conversion of arachidonic acid to prostaglandins, the mediators of the pathological effects. It’s mode of binding to COX-2 has been found to differ from the other selective COX-2 inhibitors; the carboxylic acid forms hydrogen bonds with Tyr-385 and Ser-530 in the catalytic site rather than seeking interactions within the larger hydrophobic side pocket. Since lumiracoxib is mainly metabolized by CYP2C9, a study evaluating the co-administration of lumiracoxib with fluconazole, a potent inhibitor of CYP2C9, was conducted, and it concluded that there was no need for lumiracoxib dose adjustment, since changes in the systemic exposure were not significant. No serious adverse effects were reported, but in the small number of cases where treatment was discontinued, Gastro intestinal (GI) and musculoskeletal complaints were common.
Description
Lumiracoxib is a selective inhibitor of COX-2 with IC50 values of 0.13 and 67 μM for COX-2 and COX-1, respectively, in isolated human whole blood. It reduces increases in the levels of prostaglandin E2 (PGE2; ) induced by IL-1β in human dermal fibroblasts (IC50 = 0.14 μM), as well as in LPS-stimulated RAW 264.7 cells when used at concentrations ranging from 1 to 100 μM., Lumiracoxib (3 and 10 mg/kg) also decreases LPS-induced increases in the levels of PGE2 in a rat model of air pouch inflammation. It reduces M. tuberculosis-induced increases in hind paw volume and the radiological and histopathological severity of arthritic lesions in a rat model of chronic arthritis when administered at a dose of 2 mg/kg.
Chemical properties
Pale Yellow Solid
Originator
Novartis AG (Switzerland)
The Uses of LUMIRACOXIB
Lumiracoxib is a selective cyclooxygenase-2-(COX-2) inhibitor and an anti-inflammatory agent (1,2,3,4).
The Uses of LUMIRACOXIB
Selective cyclooxygenase-2-(COX-2) inhibitor. Anti-inflammatory.
The Uses of LUMIRACOXIB
antiinflammatory, analgesic, antiarthritic
Background
Lumiracoxib is a COX-2 selective non-steroidal anti-inflammatory drug (NSAID). On August 11, 2007, Australia's Therapeutic Goods Administration (TGA, the Australian equivalent of the FDA) cancelled the registration of lumiracoxib in Australia due to concerns that it may cause liver failure. New Zealand and Canada have also followed suit in recalling the drug.
Indications
For the acute and chronic treatment of the signs and symptoms of osteoarthritis of the knee in adults.
What are the applications of Application
Lumaricoxib is a Cox-2 selective inhibitor and NSAID
Definition
ChEBI: An amino acid that is phenylacetic acid which is substituted at position 2 by the nitrogen of 2-chloro-6-fluoroaniline and at position 5 by a methyl group. A highly selective cyclooxygenase 2 inhibitor, it was briefly used for the treatment of osteoarthrit s, but was withdrawn due to concersns of hepatotoxicity.
brand name
Prexige
Biochem/physiol Actions
Lumiracoxib (COX189) is an orally active, potent and selective cyclooxygenase-2 inhibitor (Ki = 60 nM/COX-2 vs. 3.2 μM/COX-1) that inhibits COX-2-mediated PGE2 production in human whole blood (IC50 = 130 nM; stimulation = 50 μM A23187), but not COX-1-dependent TxB2 production (IC50 = 67 μM; stimulation = 10 μg/mL LPS). Lumiracoxib shows in vivo anti-inflammatory efficacy against carrageenan-induced paw oedema (ED30 = 0.35 mg/kg p.o.), CFA-induced hyperalgesia (ED30 = 5.1 mg/kg p.o.), as well as adjuvant-induced arthritis (ED50 = 3 mg/kg/day p.o.) in rats in vivo.
Pharmacokinetics
Lumiracoxib has a different structure from the standard COX-2 inhibitors (e.g. celecoxib). It more closely resembles the structure of diclofenac (one chlorine substituted by fluorine, the phenylacetic acid has another methyl group in meta position), making it a member of the arylalkanoic acid family of NSAIDs. It binds to a different site on the COX-2 receptor than the standard COX-2 inhibitors. It displays extremely high COX-2 selectivity.
Metabolism
Hepatic oxidation and hydroxylation via CYP2C9. Three major metabolites have been identified in plasma: 4'-hydroxy-lumiracoxib, 5-carboxy-lumiracoxib, and 4'-hydroxy-5-carboxy-lumiracoxib.
Properties of LUMIRACOXIB
| Melting point: | 139-141°C |
| Boiling point: | 395.7±42.0 °C(Predicted) |
| Density | 1.363±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | ≥29.4 mg/mL in DMSO; insoluble in H2O; ≥27.15 mg/mL in EtOH with ultrasonic |
| form | solid |
| pka | 4.18±0.10(Predicted) |
| color | White to light yellow |
| CAS DataBase Reference | 220991-20-8 |
Safety information for LUMIRACOXIB
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for LUMIRACOXIB
New Products
N,O-Dimethylhydroxylamine hydrochloride DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide 3-Hydroxypropionitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Lumiracoxib CAS 220991-20-8View Details
Lumiracoxib CAS 220991-20-8View Details
220991-20-8 -
![6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
2115779-15-0 -
 4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
13745-17-0 -
 230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 -
 Isopropyl amine Hydrochloride 99%View Details
Isopropyl amine Hydrochloride 99%View Details
15572-56-2 -
 19501-58-7 99%View Details
19501-58-7 99%View Details
19501-58-7 -
 Cyclopropane-1,1-dicarboxylic acid 99%View Details
Cyclopropane-1,1-dicarboxylic acid 99%View Details
598-10-7 -
 230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8
