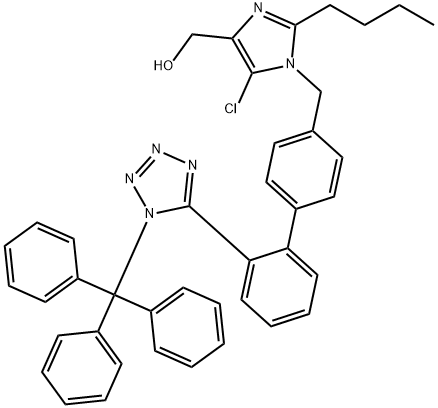
Losartan Impurity 12
- CAS NO.:727718-93-6
- Empirical Formula: C22H22ClN9
- Molecular Weight: 447.93
- Update Date: 2023-05-18 11:31:19
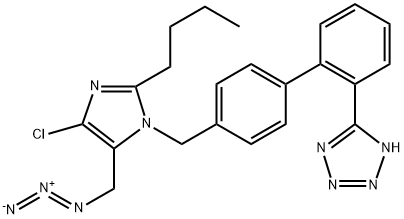
What is Losartan Impurity 12?
Description
Losartan is a medication used to treat high blood pressure. It is also used for diabetic kidney disease, heart failure, and left ventricular enlargement.Losartan was patented in 1986, and approved for medical use in the United States in 1995. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication.
Losartan was the first nonpeptide imidazole to beintroduced as an orally active angiotensin II antagonist withhigh specificity for AT1. When administered to patients, itundergoes extensive first-pass metabolism, with the 5-methanol being oxidized to a carboxylic acid. This metabolismis mediated by CYP 2C9 and 3A4 isozymes. The 5-methanol metabolite is approximately 15 times more potentthan the parent hydroxyl compound. Because the parent hydroxylcompound has affinity for the AT1 receptor, strictlyspeaking, it is not a prodrug.
The Uses of Losartan Impurity 12
Losartan Azide is an impurity of Losartan (L470470). Losartan is a nonpeptide angiotensin II AT1-receptor antagonist. Antihypertensive.
Properties of Losartan Impurity 12
| Melting point: | >139°C (dec.) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
Safety information for Losartan Impurity 12
Computed Descriptors for Losartan Impurity 12
Losartan Impurity 12 manufacturer
Clickchem Research LLP
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 727718-93-6 LOSARTAN AZIDO IMPURITY 98.62View Details
727718-93-6 LOSARTAN AZIDO IMPURITY 98.62View Details
727718-93-6 -
 727718-93-6 90 % AboveView Details
727718-93-6 90 % AboveView Details
727718-93-6 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
