L-xylose
- CAS NO.:609-06-3
- Empirical Formula: C5H10O5
- Molecular Weight: 150.13
- MDL number: MFCD00151096
- EINECS: 210-174-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:12:54
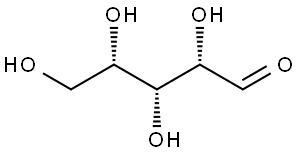
What is L-xylose?
Chemical properties
L-Xylose is a white fine crystalline powder. It is an aldopentose that has a molecular formula C5H10O5 and a molar mass of 150.13 g/mol. The structure of L-xylose is presented in Figure 1. The optical rotations of D- and L-xylose are α20D = -18.6° and α20D = +18.6°, respectively (Fischer and Ruff, 1900). L-Xylose can exist in open chain form, as a pyranose or as a furanose. The relative concentrations of different stereoisomers have not been specified for L-xylose. However, for D-xylose the distribution of different forms in water are: 36.5% α-pyranose, 6% β-pyranose and less than 1 % in open chain and furanose form (Pastinen, 2000). Most likely L-xylose acts similarly to its enantiomer, and thus pyranose rings are predominant.
Figure 1. Structure of L-xylose A) as a Fischer projection, B) as a ball and stick model in acyclic form and C) as a ball and stick model in pyranose ring form.
The Uses of L-xylose
L-Xylose is used in the synthesis of L-Xylose derivatives as selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the treatment of type 2 diabetes.
What are the applications of Application
L-(?)-Xylose is one of 2 isoforms of xylose
Definition
ChEBI: Aldehydo-L-xylose is a xylose of ring-opened form having L-configuration.
What are the applications of Application
l-Xylose is a suitable starting material for the production of L-ribonucleosides. L- Xylose can be converted into an L-ribose derivative via oxidation and reduction steps. This product is then glycosylated to give L-ribonucleosides: L-uridine, L-cytidine, L- adenosine and L-guanosine (Moyroud and Strazewski, 1999; Chelain et al., 1995).
L-Xylose can also be used as a starting material for the production of polyhydroxypyrrolidines and related analogues. These compounds have many biological activities. They are shown to have anti-HIV effects, inhibit tumor growth, and also act as ?- and ?-glucosidase inhibitors, which is of relevance for the development of diabetes drugs (Behr and Guillerm, 2007).
Preparation
L-Xylose can be produced from L-xylulose by isomerization. This can be achieved either enzymatically or chemically under alkaline conditions. The equilibrium of the reaction between D-xylose and D-xylulose in an aqueous solution has been determined at pH 6.8-7.4 and at 25 °C to be 85:15 in favor of D-xylose (Tewari et al., 1985).
Properties of L-xylose
| Melting point: | 150-152 °C(lit.) |
| Boiling point: | 191.65°C (rough estimate) |
| Density | 1.525 |
| refractive index | -20 ° (C=10, H2O) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | H2O: 0.1 g/mL, clear, colorless |
| form | Crystalline Powder |
| pka | 12.46±0.20(Predicted) |
| color | White to off-white |
| optical activity | [α]24/D 18.7°, c = 4 in H2O |
| Water Solubility | within almost transparency |
| Sensitive | Hygroscopic |
| BRN | 1723080 |
| CAS DataBase Reference | 609-06-3(CAS DataBase Reference) |
| EPA Substance Registry System | L-Xylose (609-06-3) |
Safety information for L-xylose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-xylose
| InChIKey | SRBFZHDQGSBBOR-VVZXFQNISA-N |
L-xylose manufacturer
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
![FORSKOLIN, 7BETA-DEACETYL-7BETA-[GAMMA-(MORPHOLINO) BUTYRYL]-, HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/110452-74-9.gif)
![FORSKOLIN, 6BETA-[BETA'-(PIPERIDINO)PROPIONYL]-, HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/110452-77-2.gif)

You may like
-
 L-(-)-Xylose extrapure CAS 609-06-3View Details
L-(-)-Xylose extrapure CAS 609-06-3View Details
609-06-3 -
 L-(-)-Xylose CAS 609-06-3View Details
L-(-)-Xylose CAS 609-06-3View Details
609-06-3 -
 L-Xylose CAS 609-06-3View Details
L-Xylose CAS 609-06-3View Details
609-06-3 -
 L-Xylose 98% CAS 609-06-3View Details
L-Xylose 98% CAS 609-06-3View Details
609-06-3 -
 L-(−)-Xylose, mixture of anomers CAS 609-06-3View Details
L-(−)-Xylose, mixture of anomers CAS 609-06-3View Details
609-06-3 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1
