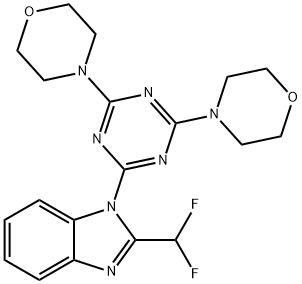
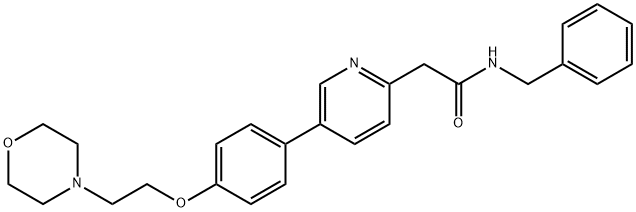
Tirbanibulin
- CAS NO.:897016-82-9
- Empirical Formula: C26H29N3O3
- Molecular Weight: 431.53
- MDL number: MFCD18633218
- EINECS: 200-258-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
What is Tirbanibulin?
Absorption
Tirbanibulin demonstrates good oral bioavailability. Following topical administration of doses ranging from 54 to 295 mg on the face or scalp, the steady-state concentration of tirbanibulin was achieved by 72 hours. At five days following initial administration, the mean Cmax was 0.34±0.30 ng/mL in subjects who received topical treatment on the face and 0.18±0.10 ng/mL in subjects who received topical treatment on the scalp. The mean AUC24 was 5.0±3.9 h x ng/mL in subjects who received topical treatment on the face and 3.2±1.9 h x ng/mL in subjects who received topical treatment on the scalp. The median Tmax was about seven hours.
Toxicity
The LD50 value of tirbanibulin has not been determined. While there is limited information on overdose from tirbanibulin, it may lead to an increase in the incidence and severity of local skin reactions.
The Uses of Tirbanibulin
KX2-391 is a Src Inhibitor with efficacy against certain hepatic and leukemia cell lines.
The Uses of Tirbanibulin
A synthetic, orally bioavailable small molecule and non-ATP competitive Src tyrosine kinase inhibitor with an IC50 of average 72 nM.
Indications
Tirbanibulin is indicated for the topical treatment of actinic keratosis on the face or scalp.
Background
Tirbanibulin (KX-O1 or KX2–391) is a dual inhibitor of Src Kinase and tubulin. On December 14, 2020, tirbanibulin was approved by the FDA for the topical treatment of actinic keratosis on the face or scalp. It is marketed under the brand name Klisyri. Actinic keratosis is a chronic condition characterized by lesions, which can potentially transform into invasive squamous cell carcinoma with a risk of 1% over 10 years. Tirbanibulin blocks the molecular pathways that promote the proliferation, survival, and metastasis of malignant cells. Tirbanibulin exhibits antitumour effects in vitro and in vivo and has been investigated for its antitumor efficacy in the management of various cancers, such as prostate cancer and breast cancer.
What are the applications of Application
KX2-391 is a Src Inhibitor with efficacy against certain hepatic and leukemia cell lines
Biological Activity
kx2-391 is a highly selective inhibitor of src kinase with ic50 value of 20nm [1].kx2-391 is a non-atp competitive inhibitor of src. it is the first inhibitor that targets src kinase within the substrate binding site. kx2-391 inhibits src catalyzed trans-phosphorylation of fak, shc, paxillin as well as src kinase autophosphorylation. kx2-391 has no effects on pdgfr, egfr, jak1, jak2 and lck demonstrating it as a selective inhibitor. it is also found to be an inhibitor of tubulin polymerization through binding to the unique confirmation on heterodimeric tubulin. in cellular assays, kx2-391 shows growth inhibition in nih3t3/c-src527f cells and syf/c-src527f cells with gi50 values of 23nm and 39nm, respectively [1, 2].since src acts as a regulator in cell proliferation survival, motility and invasiveness, kx2-391 is potent against a variety of solid tumors and many leukemia tumors. it is shown to inhibit primary tumor growth and to suppress metastasis [2].
Pharmacokinetics
In clinical trials composed comprising patients with actinic keratosis of the face or scalp, tirbanibulin promoted complete clearance of actinic keratosis lesions at day 57 in treated areas in 44-54% of patients compared to 5-13% of patients who received the placebo. Actinic keratosis is a chronic, pre-malignant condition characterized by lesions and proliferation of neoplastic keratinocytes. Tirbanibulin mediates an anti-proliferative effect by inhibiting tubulin polymerization and Src kinase signalling.
Tirbanibulin inhibited primary tumour growth and metastasis in many preclinical animal models of cancer. In human triple-negative breast cancer, or estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2)-negative tumour, xenografts, tirbanibulin suppressed tumour growth and metastasis. Tirbanibulin was also shown to restore functional ERα expression in ERα-negative breast tumours. Tirbanibulin promoted synergistic tumour growth inhibition of breast cancer cell lines when used in combination with tamoxifen and paclitaxel.
In a clinical trial comprising patients with advanced solid tumours, dose-limiting toxicities of tirbanibulin included elevated liver transaminases, neutropenia and fatigue.
Metabolism
In vitro, tirbanibulin is mainly metabolized by CYP3A4, and to a lesser extent, CYP2C8. In adult subjects with actinic keratosis, detected metabolites were KX2-5036 and KX2-5163, which were pharmacologically inactive metabolites with the highest plasma concentrations of 0.09 ng/mL and 0.12 ng/mL, respectively.
References
[1] fallah-tafti a, foroumadi a, tiwari r, et al. thiazolyl n-benzyl-substituted acetamide derivatives: synthesis, src kinase inhibitory and anticancer activities. european journal of medicinal chemistry, 2011, 46(10): 4853-4858.
[2] naing a, cohen r, dy g k, et al. a phase i trial of kx2-391, a novel non-atp competitive substrate-pocket-directed src inhibitor, in patients with advanced malignancies. investigational new drugs, 2013, 31(4): 967-973.
Properties of Tirbanibulin
| Boiling point: | 680.9±55.0 °C(Predicted) |
| Density | 1.169 |
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; ≥121 mg/mL in DMSO; ≥2.44 mg/mL in EtOH with gentle warming and ultrasonic |
| form | Powder |
| pka | 14.73±0.46(Predicted) |
| color | White to off-white |
Safety information for Tirbanibulin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tirbanibulin
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8