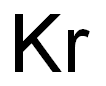Krypton(II) difluoride
- CAS NO.:13773-81-4
- Empirical Formula: F2Kr
- Molecular Weight: 121.8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-31 14:38:37
What is Krypton(II) difluoride?
Description
The elements in group 18 of the periodic table (the “noble gases”) were once considered to be chemically inert. But over the years, chemists discovered how to make molecules that contain them.
In the case of krypton, J. J. Turner and G. C. Pimentel at the University of California, Berkeley, used electrical discharge to prepare krypton difluoride (KrF2) in 1963. The colorless solid decomposes at room temperature, but it can be stored indefinitely at –78 oC.
KrF2?is an extremely strong oxidizing and fluorinating agent. It can convert metallic gold to AuF5, metallic silver to AgF3, and xenon to XeF6. It can also oxidize chlorine and bromine to their +5 oxidation states.
Chemical properties
colorless solid; decomposes rapidly at room temp; tetr, a=0.6533 nm, c=0.5831 nm; vapor pressure at 0°C is 29mm Hg; thermodynamically unstable, can be stored at ?78°C; prepared by electrical discharge of Kr and F2 at ?183°C; used in certain types of electric light bulbs [MER06] [KIR78] [COT88]
Physical properties
White tetragonal crystal; sublimes under vacuum at 0°C; decomposes slowly at 25°C; density 3.24 g/cm3; reacts with water; vapor pressure 30 torr at 0°C; slightly soluble in liquid fluorine.
The Uses of Krypton(II) difluoride
As gas filler in special purpose electric bulbs. To initiate and sustain the arc in metal-vapor discharge tubes, such as mercury and sodium vapor lamps. To determine surface areas of fine solids by adsorption techniques.
Preparation
Krypton difluoride may be prepared by the reaction of krypton with fluorine in an electric discharge at low pressure and liquid oxygen temperature. Also it may be made by irradiating krypton with ultraviolet rays in a fluorine—argon gas mixture at liquid helium temperature (-196°F).
Kr + F2 → KrF2
The product can be stored at -78°C without decomposition
.
Properties of Krypton(II) difluoride
| Melting point: | decomposes at ~25℃ [KIR78] |
| Density | (calc) 3.24 |
| solubility | reacts with H2O |
| form | colorless tetragonal crystals |
| color | colorless tetragonal crystals, crystalline |
| EPA Substance Registry System | Krypton fluoride (KrF2) (13773-81-4) |
Safety information for Krypton(II) difluoride
Computed Descriptors for Krypton(II) difluoride
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1