KB-0742 dihydrochloride
- CAS NO.:2416874-75-2
- Empirical Formula: C16H26ClN5
- Molecular Weight: 323.87
- MDL number: MFCD32899897
- Update Date: 2026-01-20 19:14:41
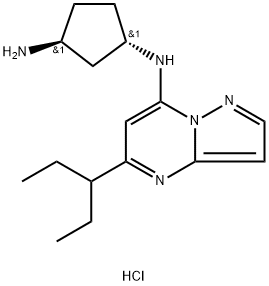
What is KB-0742 dihydrochloride?
Biological Activity
KB-0742 dihydrochloride is a potent, selective and orally active CDK9 inhibitor with an IC50 of 6 nM for CDK9/cyclin T1. KB-0742 dihydrochloride is selective for CDK9/cyclin T1 with >50-fold selectivity over other CDK kinases. KB-0742 dihydrochloride has potent anti-tumor activity[1]. KB-0742 (6 hours; 0.1-10 μM; 22Rv1 cells) treatment significant reduction of downstream phosphorylation of RNA Pol II at Ser2 and Ser7, and diminished phosphorylation at Ser5. Global androgen receptor (AR)-FL and AR-V protein levels are significantly reduced starting at 6 h treatment time, which is accompanied by the reduction of phospho-AR levels (Ser81)[1].KB-0742 (48-72 hours) treatment shows cytostatic effects in prostate cancer and leukemia cell lines. KB-0742 shows antiproliferative activity with GR50s of 0.183 μM and 0.288 μM for 22Rv1 cells and MV-4-11 AML cells, respectively[1].In 22Rv1 cells, KB-0742 rapidly downregulates nascent transcription, preferentially depleting short half-life transcripts and AR-driven oncogenic programs[1]. KB-0742 (3-30 mg/kg; p.o.; daily; over 21 days) is well tolerated even at high dose, while significantly reducing tumor burden in 22Rv1 human prostate cancer cell line-derived xenograft (CDX) models[1].
References
[1]. André Richters, et al. Modulating Androgen Receptor-Driven Transcription in Prostate Cancer with Selective CDK9 Inhibitors. Cell Chem Biol. 2020 Oct 20;S2451-9456(20)30380-9.
Properties of KB-0742 dihydrochloride
| storage temp. | 4°C, away from moisture |
| solubility | |DMSO : 62.5 mg/mL (173.45 mM; Need ultrasonic) |
| form | Solid |
| color | Light yellow to yellow |
| Water Solubility | Water : 100 mg/mL (277.52 mM; Need ultrasonic) |
Safety information for KB-0742 dihydrochloride
Computed Descriptors for KB-0742 dihydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
