K-252A
- CAS NO.:99533-80-9
- Empirical Formula: C27H21N3O5
- Molecular Weight: 467.47
- MDL number: MFCD09878276
- EINECS: 640-127-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-22 22:14:22
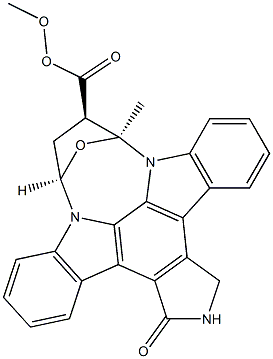
What is K-252A?
Description
K252a is a staurosporine analog isolated from Nocardiopsis sp. soil fungi that inhibits protein kinase (PK) C, PKA, Ca2+/calmodulin-
The Uses of K-252A
K-252a is used to inhibit protein kinases involved in cell signaling processes, including CaM kinase; phosphorylase kinase, serine/threonine kinases, Trk. It is used to inhibit the actions of nerve growth factor (NGF) on PC1 2 cells and to inhibit smooth muscle myosin light chain kinase .
What are the applications of Application
K-252a is A cell permeable protein kinase inhibitor
Definition
ChEBI: A organic heterooctacyclic compound that is a potent inhibitor of protein kinase C and is isolated from Nocardiopsis sp K-252a
Biological Activity
Non-selective protein kinase inhibitor; analog of staurosporine. Inhibits PKC (IC 50 = 32.9 nM), Ca 2+ /calmodulin-stimulated phosphodiesterases (IC 50 = 1.3-2.9 μ M), MLCK (K i = 20 nM) and receptor tyrosine kinases. Inhibits the oncogenic properties of MET; prevents autophosphorylation and activation of downstream effectors (MAPK, Akt).
Biochem/physiol Actions
Potent inhibitor of various protein kinases including protein kinase A, C, and G. It is is reported to selectively inhibit the actions of nerve growth factor (NGF) on PC1 2 cells and has been shown to inhibit smooth muscle myosin light chain kinase .
Storage
-20°C
References
1) Kase et al. (1987), K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases; Biochem. Biophys. Res. Commun., 142 436 2) Hashimoto et al. (1991), Potent and preferential inhibition of Ca2+/calmodulin-dependent protein kinase II by K252a and its derivative KT5926; Biochem. Biophys. Res. Commun., 181 423 3) Berg et al. (1992), K-252a inhibits nerve growth factor-induced trk proto-ooncogene tyrosine phosphorylation and kinase activity; J. Biol. Chem., 267 13 4) Ruegg et al. (1989), Staurosporine, K-252 and UCN-01: potent but non-specific inhibitors of protein kinases; Trends Pharmacol. Sci., 10 218 5) Mohri et al. (1999), K252a induces cell cycle arrest and apoptosis by inhibiting Cdc2 and Cdc25c; Cancer Invest., 17 391
Properties of K-252A
| Melting point: | 262-273℃ (decomposition) (methanol ) |
| Boiling point: | 685.3±55.0 °C(Predicted) |
| Density | 1.68 |
| Flash point: | 87℃ |
| storage temp. | -20°C |
| solubility | DMF: 1 mg/mL |
| form | Lyophilized solid. |
| pka | 11.95±0.40(Predicted) |
| color | White or off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. |
Safety information for K-252A
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H371:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for K-252A
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran





You may like
-
 K-252a CAS 99533-80-9View Details
K-252a CAS 99533-80-9View Details
99533-80-9 -
 K-252a CAS 99533-80-9View Details
K-252a CAS 99533-80-9View Details
99533-80-9 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
