JNK-IN-8
Synonym(s):3-[[4-(Dimethylamino)-1-oxo-2-buten-1-yl]amino]-N-[3-methyl-4-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-benzamide
- CAS NO.:1410880-22-6
- Empirical Formula: C29H29N7O2
- Molecular Weight: 507.59
- MDL number: MFCD22124890
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-27 08:46:37
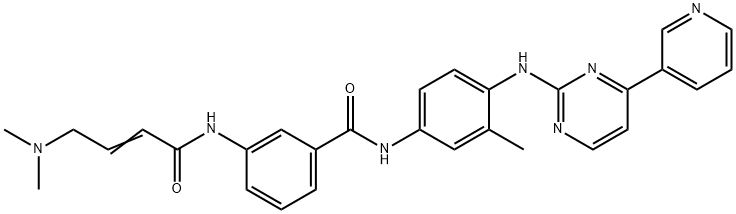
What is JNK-IN-8?
The Uses of JNK-IN-8
JNK-IN-8 has been used as an inhibitor to address the importance of JNK signaling in withaferin A (WFA)-induced apoptosis of myelodysplastic syndromes (MDS)-L cells.
What are the applications of Application
JNK Inhibitor XVI is a selective and irreversible type 2 inhibitor of JNK
Definition
ChEBI: 3-[[4-(dimethylamino)-1-oxobut-2-enyl]amino]-N-[3-methyl-4-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide is a member of benzamides.
Biological Activity
jnk-in-8 is a specific jnk1/2/3 inhibitor with ic50 value of 4.67, 18.7, 0.98 nm respectively [1].c-jun n-terminal kinase (jnk) 1, 2 and 3 belong to the mitogen-activated protein kinase (mapk) family, which are able to phosphorylate c-jun on the ser63 and ser73 residue.they are responsive for stress stimuli, including cytokines and heat shock, and get involved in t cell differentiation and cell apoptosis process. jnk 1 and 2 are ubiquitous in all cell types but jnk 3 is only found in cells of brain, heart and testes tissues.jnk-in-8 is a jnk1/2/3 inhibitor with high specificity. when jnk-in-8 was profiled with a panel of 400 kinases, it exhibited specific binding to jnk 1/2/3 but not to other kinases. crystallization study also found that jnk-in-8 forms covalent bonds with conserved cysteine residue of jnk 1/2/3, resulting in a conformational change of the activation loop that blocks the substrate binding, thereby inhibiting the activity of jnk 1/2/3 [1].in hela cells and a375 cells, pretreatment of cells with jnk-in-8 resulted in the inhibition of c-jun which is a direct phosphorylation substrate of jnk 1/2/3, confirming the inhibitory action of jnk-in-8 on jnk 1/2/3. in hek293-ilr1 cells following stimulation by anisomycin, the jnk-in-8 was observed to inhibit c-jun but not msk1 and p38, and the inhibition was not reversible by removing jnk-in-8 from culture medium. additionally, jnk-in-8 only exhibited on-pathway inhibition of jnk signaling pathway, which can be monitored by the phosphorylation of c-jun [1].
Biochem/physiol Actions
JNK-IN-8 is a potent, selective and irreversible inhibitor of JNK1/2/3 that inhibits phosphorylation of c-Jun. JNK-IN-8 forms covalent bonds with a conserved cysteine residue.
References
[1]. zhang t et al., discovery of potent and selective covalent inhibitors of jnk. chemical biology. 2012, 19(1):140-154.
Properties of JNK-IN-8
| Density | 1.283±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | ≥25.4 mg/mL in DMSO; insoluble in H2O; ≥9.24 mg/mL in EtOH with gentle warming and ultrasonic |
| pka | 13.34±0.70(Predicted) |
| form | powder |
| color | white to beige |
Safety information for JNK-IN-8
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for JNK-IN-8
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![N-(3-Cyano-4,5,6,7-tetrahydrobenzo[b]thienyl-2-yl)-1-naphthalenecarboxamide](https://img.chemicalbook.in/CAS/20150408/GIF/312917-14-9.gif)

You may like
-
 1410880-22-6 JNK-IN-8 Only for R&D 98%View Details
1410880-22-6 JNK-IN-8 Only for R&D 98%View Details
1410880-22-6 -
 JNK-IN-8 95.00% CAS 1410880-22-6View Details
JNK-IN-8 95.00% CAS 1410880-22-6View Details
1410880-22-6 -
 JNK-IN-8 CAS 1410880-22-6View Details
JNK-IN-8 CAS 1410880-22-6View Details
1410880-22-6 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
