IWP-3
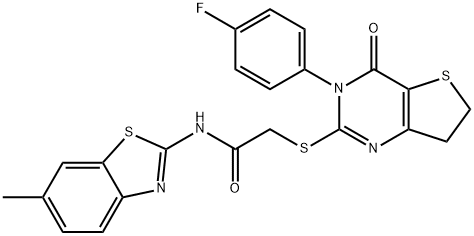
Synonym(s):2-[[3-(4-Fluorophenyl)-3,4,6,7-tetrahydro-4-oxothieno[3,2-d]pyrimidin-2-yl]thio]-N-(6-methyl-2-benzothiazolyl)-acetamide
- CAS NO.:687561-60-0
- Empirical Formula: C22H17FN4O2S3
- Molecular Weight: 484.59
- MDL number: MFCD04457889
- EINECS: 200-258-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-10 14:44:25
What is IWP-3?
The Uses of IWP-3
IWP-3 is a potent inhibitor for porcupine which is known to catalyze the palmitoylation of Wnt proteins which are crucial in tumorigenesis.
Biological Activity
iwp-3 is an inhibitor of wnt production.wnt signaling proteins, a group of small secreted proteins, are active in tissue homeostasis, embryonic development, as well as tumorigenesis. wnt proteins can bind to the cell surface receptors, initiating a signaling cascade that results in the β-catenin activation of gene transcription.
Biochem/physiol Actions
IWP-3 is a Wnt inhibitor that prevents palmitylation of Wnt proteins by Porcupine (Porcn). IWP-3 is an inactivator of Porcn function that acts as inhibitors of Wnt production. Studies revealed that IWP-3 induces differentiation of human mesoderm cells to form cardiomyocytes.
in vitro
in previous study, iwp-3 was identified as an inhibitor of wnt production that could regulate in vitro wnt pathway activity with an ic50 value of 40 nm. in addition, iwp-3 was found to be able to inactivate porcupine, a o-acyltransferase for palmitoylating wnt proteins that was critical for the signaling ability and secretion. moreover, iwp-3 at 5 μm was found to block wnt-dependent phosphorylation of the low-density lipoprotein receptor-related protein 6 and the scaffold protein dishevelled, resulting in preventing the accumulation of β-catenin [1]. in another study, iwp-3 was applied to promote cardiomyocyte generation from human embryonic stem cells by inhibiting wnt signaling [2].
References
[1] b. chen, m. e. dodge, w. tang, et al. small molecule-mediated disruption of wnt-dependent signaling in tissue regeneration and cancer. nature chemical biology 5(2), 100-107 (2009).
[2] e. willems, s. spiering, h. davidovics, et al. small molecule inhibitors of the wnt pathway potently promote cardiomyocytes from human embryonic stem cell derived mesoderm. circulation research 109(4), 360-364 (2011).
Properties of IWP-3
| Melting point: | >248°C (dec.) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble2mg/mL (clear solution, warmed) |
| form | powder |
| color | white to beige |
Safety information for IWP-3
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for IWP-3
New Products
tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-Arg-OH HCl H2O Indole Methyl Resin 2-CTC Resin Gabapentin EP Impurity A 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 1,3-Diphenylurea 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID N-METHYL INDAZOLE-3-CARBOXYLIC ACID 2-Hydroxy-5-nitroacetophenone 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2-HYDROXY BENZYL CYANIDE DIETHYL AMINOMALONATE HYDROCHLORIDE 5-BROMO-2CYANO PYRIDINE Boldenone Clarithromycin Ethinyl Estradiol StanozololYou may like
-
 CIS BROMO BENZOATE 98%View Details
CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 3-NITRO 2-METHYL BENZOIC ACID 98%View Details
3-NITRO 2-METHYL BENZOIC ACID 98%View Details
1975-50-4 -
 INDAZOLE-3-CARBOXYLIC ACID 4498-67-3 98%View Details
INDAZOLE-3-CARBOXYLIC ACID 4498-67-3 98%View Details
4498-67-3 -
 221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 98%View Details
221615-75-4 -
![(6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE 98%View Details
(6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE 98%View Details
0/2/39 -
 2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 MELDRUMS ACID 98%View Details
2033-24-1 -
 42831-50-5 98%View Details
42831-50-5 98%View Details
42831-50-5
