ISX 9
Synonym(s):Isx, N-Cyclopropyl-5-(thiophen-2-yl)isoxazole-3-carboxamide;N-Cyclopropyl-5-(thiophen-2-yl)isoxazole-3-carboxamide, Isx;Neuronal Differentiation Inducer III - CAS 832115-62-5 - Calbiochem
- CAS NO.:832115-62-5
- Empirical Formula: C11H10N2O2S
- Molecular Weight: 234.27
- MDL number: MFCD04133937
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-16 16:10:54
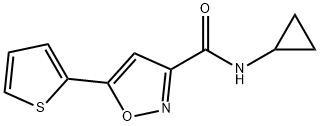
What is ISX 9?
Description
ISX9 (832115-62-5) promotes neurogenesis in vivo enhancing the proliferation and differentiation of hippocampal subgranular zone neuroblasts and enhances memory.1?Induces robust neuronal differentiation in adult neural stem cells.2?Increases insulin production by pancreatic β cells.3?ISX9 blocks malignant astrocyte proliferation, downregulates their astrocyte character, induces reentry into the cell cycle and upregulates neuronal gene expression.4 Induces sensory neurons from neuroepithelial stem cells.5 Potentiates cell proliferation and neuronal commitment in the rat dentate gyrus.6
The Uses of ISX 9
N-Cyclopropyl-5-(thiophen-2-yl)isoxazole-3-carboxamide was shown to exhibit regulatory effects towards the expression of pH sensing G protein-coupled receptor-68 and is now a new drug target for ischemic heart disease.
What are the applications of Application
Neuronal Differentiation Inducer III is a neuronal differentiation inducing compound
Definition
ChEBI: N-cyclopropyl-5-thiophen-2-yl-3-isoxazolecarboxamide is a heteroarene and an aromatic amide.
General Description
A cell-permeable isoxazole compound that selectively induces robust neuronal differentiation (Effective concentration = 20 μM) in various stem/progenitor cells. Induces insulin expression, and activates ERK signaling and histone acetylation in MIN6 pancreatic β cells. The underlying excitation-neurogenesis signaling is comprised of, at least in part, an initial NMDA receptor- and L-type chanenels-mediated Ca2+ influx, followed by an activation of CaMKII, phosphorylation/nuclear export of the MEF2 repressor HDAC5, the subsequent de-repression of MEF2, and the eventual activation of the HDAC-MEF2 epigenetic/transcriptional regulatory network.
Storage
+4°C
References
1) Petrik et al. (2012), Functional and mechanistic exploration of an adult neurogenesis-promoting small molecule; FASEB J., 26 3148 2) Schneider et al. (2008), Small-molecule activation of neuronal cell fate; Nat. Chem. Biol., 4 408 3) Dioum et al. (2011), A small molecule differentiation inducers increases insulin production by pancreatic β cells; Proc. Natl. Acad. Sci. USA, 108 20713 4) Zhang et al. (2011), Small molecule blocks malignant astrocyte proliferation and induces neuronal gene expression; Differentiation, 81 233 4) Ali et al. (2016) Induction of sensory neurons from neuroepithelial stem cells by the ISX9 small molecule; Am. J. Stem. Cells 5 19 5) Bettio et al. (2016) ISX-9 can potentiate cell proliferation and neuronal commitment in the rat dentate gyrus; Neuroscience, 332 212
Properties of ISX 9
| Boiling point: | 468.1±35.0 °C(Predicted) |
| Density | 1.39±0.1 g/cm3(Predicted) |
| storage temp. | -20° |
| solubility | Soluble in DMSO (up to 60 mg/ml) or in Ethanol (up to 16 mg/ml). |
| form | solid |
| pka | 13.60±0.20(Predicted) |
| color | Tan |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for ISX 9
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ISX 9
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 ISX >95% CAS 832115-62-5View Details
ISX >95% CAS 832115-62-5View Details
832115-62-5 -
 Neuronal Differentiation Inducer III CAS 832115-62-5View Details
Neuronal Differentiation Inducer III CAS 832115-62-5View Details
832115-62-5 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5