Ferrous Glycine Sulphate
- CAS NO.:14729-84-1
- Empirical Formula: C2H5NO2.1/2Fe.1/2H2O4S
- Molecular Weight: 302.0408
- EINECS: 2387843
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-04 14:31:13
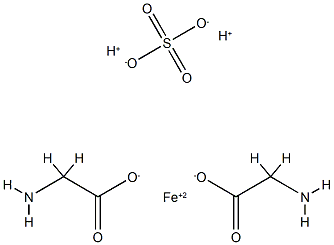
What is Ferrous Glycine Sulphate?
Absorption
The efficiency of absorption depends on the salt form, the amount administered, the dosing regimen and the size of iron stores. Subjects with normal iron stores absorb 10% to 35% of an iron dose. Those who are iron deficient may absorb up to 95% of an iron dose.
Toxicity
Acute iron overdosage can be divided into four stages. In the first stage, which occurs up to six hours after ingestion, the principal symptoms are vomiting and diarrhea. Other symptoms include hypotension, tachycardia and CNS depression ranging from lethargy to coma. The second phase may occur at 6-24 hours after ingestion and is characterized by a temporary remission. In the third phase, gastrointestinal symptoms recur accompanied by shock, metabolic acidosis, coma, hepatic necrosis and jaundice, hypoglycemia, renal failure and pulmonary edema. The fourth phase may occur several weeks after ingestion and is characterized by gastrointestinal obstruction and liver damage. In a young child, 75 milligrams per kilogram is considered extremely dangerous. A dose of 30 milligrams per kilogram can lead to symptoms of toxicity. Estimates of a lethal dosage range from 180 milligrams per kilogram and upwards. A peak serum iron concentration of five micrograms or more per ml is associated with moderate to severe poisoning in many.
The Uses of Ferrous Glycine Sulphate
Ferrous glycine sulphate, an organic complex of glycine and ferrous sulphate, is an iron supplement indicated in the treatment of iron deficiency and iron deficiency anemia.
Indications
Used in preventing and treating iron-deficiency anemia.
Pharmacokinetics
The major activity of supplemental iron is in the prevention and treatment of iron deficiency anemia. Iron has putative immune-enhancing, anticarcinogenic and cognition-enhancing activities.
Metabolism
Not Available
Safety information for Ferrous Glycine Sulphate
Ferrous Glycine Sulphate manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileYou may like
-
 Powder Ferrous Glycine SulphateView Details
Powder Ferrous Glycine SulphateView Details
17169-60-7 -
 Ferrous Glycine Sulphate - Capsule Grade / Liquid GradeView Details
Ferrous Glycine Sulphate - Capsule Grade / Liquid GradeView Details
17169-60-7 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
