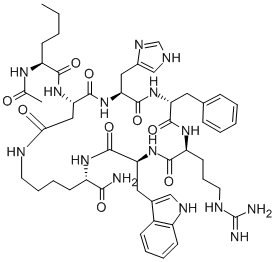
Ipamorelin
Synonym(s):2-Methylalanyl-L-histidyl-3-(2-naphthalenyl)-D-alanyl-D-phenylalanyl-L-lysinamide acetate;Aib-His-D-2-Nal-D-Phe-Lys-NH2 acetate;NNC 26-0161 acetate;
- CAS NO.:170851-70-4
- Empirical Formula: C38H49N9O5
- Molecular Weight: 711.85
- MDL number: MFCD02179658
- EINECS: 227-005-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-19 18:11:36
What is Ipamorelin?
Description
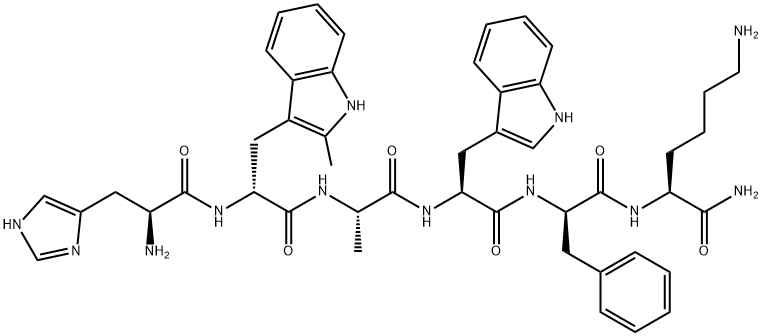
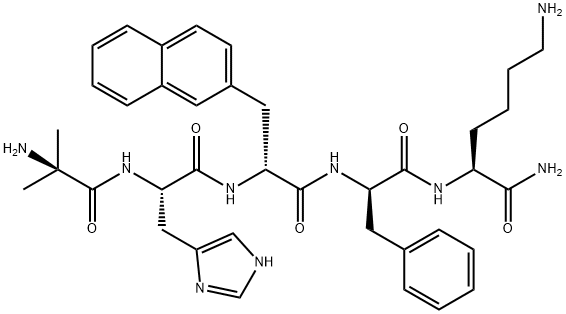
Ipamorelin (INN) (developmental code name NNC 26-0161) is a peptide selective agonist of the ghrelin/growth hormone secretagogue receptor (GHS) and a growth hormone secretagogue.It is a pentapeptide with the amino acid sequence Aib-His-D-2-Nal-D-Phe-Lys-NH2 that was derived from GHRP-1.
The Uses of Ipamorelin
Ipamorelin was originally developed by Novo Nordisk, and was investigated in phase II clinical trials by Helsinn Therapeutics for the treatment of postoperative ileus, but was discontinued due to lack of efficacy.Ipamorelin has been used by athletes as a performance enhancing drug.
The Uses of Ipamorelin
Ipamorelin is a metabolite of growth hormone releasing peptides (GHRPs). Ipamorelin is a pentapeptide with strong growth hormone releasing potency that evokes insulin release from pancrease of normal and diabetic rats.
Benefits
Ipamorelin has a wide range of benefits for the human body such as: reduced body fat, increased collagen production, increased lean muscle mass, improved sleep, increased cell repair and regeneration, elevated IGF-1, increased bone mineral content, counteracted glucocorticoid catabolism, decreased appetite stimulation compared to GHRP-6, decreased release of cortisol, prolactin and aldosterone. Decreased release of cortisol, prolactin and aldosterone.
Side Effects
Ipamorelin is generally considered safe and well tolerated, with common side effects including dizziness, headache, nausea, diarrhoea and flushing. Allergic or serious adverse reactions are rare.
Properties of Ipamorelin
| Melting point: | 144-147°C |
| Boiling point: | 1185.5±65.0 °C(Predicted) |
| Density | 1.263±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Acetonitrile (Slightly, Heated), Methanol (Very Slightly), Water (Slightly) |
| form | Solid |
| pka | 13.04±0.46(Predicted) |
| color | White to Off-White |
Safety information for Ipamorelin
Computed Descriptors for Ipamorelin
| InChIKey | NEHWBYHLYZGBNO-BVEPWEIPSA-N |
| SMILES | C(N)(=O)[C@H](CCCCN)NC(=O)[C@@H](CC1=CC=CC=C1)NC(=O)[C@@H](CC1=CC=C2C(=C1)C=CC=C2)NC(=O)[C@H](CC1N=CNC=1)NC(=O)[C@](C)(C)N |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 Ipamorelin 98% (HPLC) CAS 170851-70-4View Details
Ipamorelin 98% (HPLC) CAS 170851-70-4View Details
170851-70-4 -
 Ipamorelin acetate CAS 170851-70-4View Details
Ipamorelin acetate CAS 170851-70-4View Details
170851-70-4 -
 Ipamorelin 2mg 5mg 10mg Peptide, Injection, Packaging Type: BoxView Details
Ipamorelin 2mg 5mg 10mg Peptide, Injection, Packaging Type: BoxView Details
170851-70-4 -
 Ipamorelin 10 mg, For PersonalView Details
Ipamorelin 10 mg, For PersonalView Details
170851-70-4 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8