Imidafenacin
- CAS NO.:170105-16-5
- Empirical Formula: C20H21N3O
- Molecular Weight: 319.4
- MDL number: MFCD09833703
- EINECS: 689-703-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
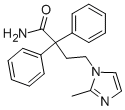
What is Imidafenacin?
Description
Imidafenacin, an M3/M1 muscarinic receptor antagonist, was introduced in
Japan for the oral treatment of OAB. The majority of OAB symptoms are
thought to result from overactivity of the detrusor muscle, which is primarily
mediated by acetylcholine-induced stimulation of muscarinic M3 receptors in the
bladder. Previously marketed muscarinic antagonists for OAB include propiverine,
tolterodine, oxybutynin, trospium, darifenacin, and solifenacin. In vitro,
imidafenacin is equally active against M1 and M3 receptors (Kb=0.32 and
0.55nM, respectively), and approximately 10-fold less active against M2 receptors
(Kb=4.13nM).
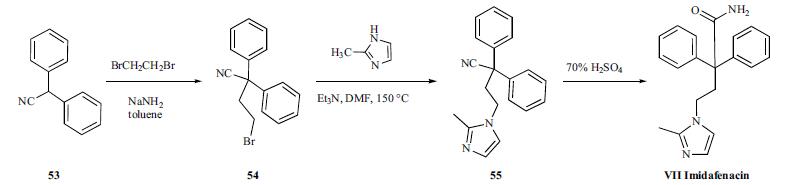
Imidafenacin is chemically synthesized in three steps starting with alkylation of diphenylacetonitrile with dibromoethane, followed by condensation with 2-methylimidazole, and hydrolysis of the cyano group to a carboxamide group with 70% sulfuric acid.
Chemical properties
White Solid
Originator
Kyorin (Japan)
The Uses of Imidafenacin
Imidafenacin-d10 is deutirium labelled imidafenacin which is a novel therapeutic agent for overactive bladder with antimuscarinic activity, on mediator release from urothelium and detrusor overactivity induced by cerebral infarction.
The Uses of Imidafenacin
A novel therapeutic agent for overactive bladder with antimuscarinic activity, on mediator release from urothelium and detrusor overactivity induced by cerebral infarction. A muscarinic antagonist.
Definition
ChEBI: Imidafenacin is a diarylmethane.
brand name
Staybla
Mechanism of action
Imidafenacin binds to and antagonizes muscarinic M1 and M3 receptors with high affinity. It also antagonizes muscarinic M2 receptors but with lower affinity. M3 receptors stimulate the contraction of the detrusor muscle in the bladder via the release of calcium from the sarcoplasmic reticulum. M2 receptors are also present in the detrusor muscle but inhibit adenylate cyclase, which reduces the relaxation mediated by β adrenergic receptors. Finally, M1 receptors are present in the parasympathetic neurons, which release acetylcholine in the bladder. They act as an autocrine positive feedback loop and further increase the release of acetylcholine. Antagonism of these receptors by imidafenacin prevents contraction of the bladder's detrusor muscle, prevents inhibition of the relation produced by sympathetic tone, and reduces acetylcholine release. Together, these reduce the frequency of urination.
Side Effects
Like all medicines, imidafenacin can cause side effects, but not everybody will experience them. This drug can cause sleepiness, blurred vision, stomach upset, constipation, and increased triglyceride levels.
Synthesis
Diphenylacetonitrile (53) was alkylated with dibromoethane in the presence of NaNH2 in toluene to give bromide compound 54. The bromide 54 was condensed with 2-methylimidazole in the presence of Et3N in hot DMF to afford 2-methylimidazole derivative 55. Hydrolysis of the cyano group of 55 with 70% sulfuric acid provided imidafenacin (VII).
Properties of Imidafenacin
| Melting point: | 184-187°C |
| Boiling point: | 579.7±50.0 °C(Predicted) |
| Density | 1.12±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO, Methanol |
| form | Solid |
| pka | 15.72±0.50(Predicted) |
| color | White |
| InChI | InChI=1S/C20H21N3O/c1-16-22-13-15-23(16)14-12-20(19(21)24,17-8-4-2-5-9-17)18-10-6-3-7-11-18/h2-11,13,15H,12,14H2,1H3,(H2,21,24) |
Safety information for Imidafenacin
Computed Descriptors for Imidafenacin
| InChIKey | SQKXYSGRELMAAU-UHFFFAOYSA-N |
| SMILES | N1(CCC(C2=CC=CC=C2)(C2C=CC=CC=2)C(N)=O)C=CN=C1C |
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Imidafenacin 98% (HPLC) CAS 170105-16-5View Details
Imidafenacin 98% (HPLC) CAS 170105-16-5View Details
170105-16-5 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
