ibritumomab tiuxetan
- CAS NO.:206181-63-7
- Empirical Formula: C24H35N5O11
- Molecular Weight: 569.5616
- Update Date: 2022-12-21 16:56:50
What is ibritumomab tiuxetan?
Description
Radioimmunotherapy (RIT) is a new treatment modality for B-cell non-Hodgkin’s lymphoma (NHL). The goal of RIT is to deliver ionizing radiation selectively to tumors while minimizing radiation absorbed in normal tissues.90 Y-lbntumomab tiuxetan is the first commercially available radiolabeled antibody for cancer therapy and more specifically for the treatment of relapsed or refractory low-grade, follicular, or transformed B-cell NHL including patients with rituximab-refractory follicular NHL. lbritumomab is a murine immunoglobulin Gl kappa isotype monoclonal antibody produced in Chinese hamster ovary cells. It targets CD20, a β-lymphocyte antigen. The purified antibody is subsequently reacted with the isothiocyanatobenzyl derivative of DTPA to form ibritumomab tiuxetan. Tiuxetan forms a stable covalent urea type bond with the antibody and can chelate a radionuclide via its five carboxyl groups: either indium-111 for imaging (medium energy gamma emitter) or yttrium-90 for radiotherapy (pure high-energy beta-emitter, mean=0.94 MeV). Rituximab (Rituxane? MabThera) is an unlabeled chimeric antibody also directed against CD20. The ZevalinTM therapeutic regimen starts with the imaging protocol: infusion of 250 mg/m2 rituximab to clear peripheral B-cells and improve targeting of radioisotope to tumor cells, followed by 5 mCi 111In-ZevalinTM for whole body imaging to enabie determination of favorable biodistribution of radiolabeled antibody. The therapeutic dose (0.3-0.4 mCi/kg) of 90Y-ZevalinTM is delivered on dfjs 7-9 following another predosing of 250 mg/m2 rituximab. The pure beta-emitting 90Y can be given with few radiation precautions. It has a long path length ‘X90=5 mm) allowing the delivery of a cytotoxic radiation dose to tumor cells more distant to the antibody-bound cell. Its short half-life (64 h) approximates the biological half-life of the radiolabeled antibody (47 h) which may minimize radiotoxicity to nontarget organs. The non tumor distribution is primarily to the bone. In a phase III clinical trial of 143 patients with relapsed or refractory low-grade, follicular, or CD20-positive transformed B-cell NHL, ZevalinTM combined with Rituxan? showed an overall response rate (ORR) of 80%, compared to Rituxan? alone which gave an ORR of 56%. Also, 30% of ZevalinTM-treated patients achieved complete responses compared to 16% of Rituxan? patients. .
Originator
IDEC (US)
The Uses of ibritumomab tiuxetan
Antineoplastic (monoclonal antibody) [Note—Yttrium-labeled ibritumomab tiuxetan is used for the treatment of non-Hodgkin’s B-cell lymphoma, coadministered with rituximab].
brand name
Zevalin
General Description
Ibritumomab (Zevalin kits to prepare In-111 Zevalin and Y-90 Zevalin, murine) is an MAb derived from an initial sensitizationwith CD20 antigen, expressed on the surface ofnormal and malignant B cells. The antibody is a murineIgG1 κ subtype, directed against CD20 antigen. It is producedin a CHO cell line. Ibritumomab is indicated for useas a multistage regimen to treat patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin lymphoma, including patients with rituximabrefractoryfollicular non-Hodgkin lymphoma.
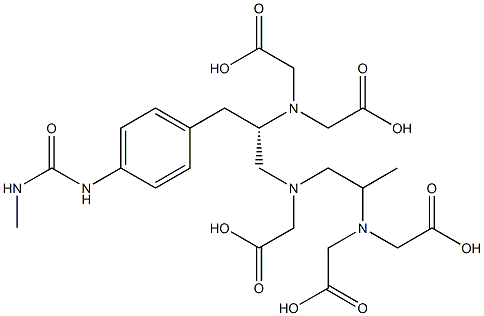
Ibritumomab tiuxetan binds specifically to CD20 antigen(human B-lymphocyte–restricted differentiation antigen).CD20 is expressed on pre-B and mature B lymphocytes andon more than 90% of B-cell non-Hodgkin lymphoma. Whenthe CDR of ibritumomab tiutuxan binds to the CD20 antigen,apoptosis is initiated. The tiutuxan chelate binds indium-111and yttrium-90 tightly. Beta emission induces cellular damageby forming free radicals in the target cells and neighboringcells. Tiutuxan is [N-[2-bis(carboxymethyl)amino]-3-(p-isothiocyanatophenyl)propyl]-[N-[2-bis(carboxymethyl)amino]2-(methyl)-ethyl]glycine.
Safety information for ibritumomab tiuxetan
New Products
Methyl 2-hydroxy-3-nitrobenzoate 1,3-Diethyl-1,3-Diphenylurea 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 1,3-Di Iodo Benzene Methyl 2-oxo-2,3-dihydrobenzo[d]oxazole-7-carboxylate 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 3-Hydroxy-4-nitrobromobenzene 2-Ethyl-1,4-diaminobenzene 2-Ethylhexyl 4-aminobenzoate Thio AcetamideYou may like
-
 MFCD22560726 98%View Details
MFCD22560726 98%View Details
MFCD22560726 -
 3-Hydroxy-4-nitrobromobenzene 98%View Details
3-Hydroxy-4-nitrobromobenzene 98%View Details
2768-84-0 -
 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 98%View Details
4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 98%View Details
1234064-08-4. -
 2-Ethyl-1,4-diaminobenzene 98%View Details
2-Ethyl-1,4-diaminobenzene 98%View Details
MFCD19203885 -
 85-81-4 6-Methoxy-8-nitroquinoline 98%View Details
85-81-4 6-Methoxy-8-nitroquinoline 98%View Details
85-81-4 -
 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 98%View Details
2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 98%View Details -
 Hot Sales:Thio AcetamideView Details
Hot Sales:Thio AcetamideView Details
62-55-5 -
 CosmoticsView Details
CosmoticsView Details
26218-04-2
