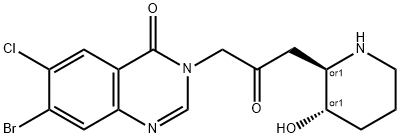
Halofuginone hydrobromide
Synonym(s):trans-(±)-7-Bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]-4(3H)-quinazolinone monohydrobromide
- CAS NO.:64924-67-0
- Empirical Formula: C16H17BrClN3O3.HBr
- Molecular Weight: 495.59
- MDL number: MFCD00946455
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-29 15:22:00
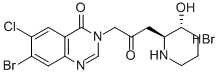
What is Halofuginone hydrobromide?
Description
Halofuginone hydrobromide (Halofuginone) is a specific collagen Type I inhibitor that antagonize or inhibit the development of new blood vessels, hence can prevent intimal hyperplasia at a vascular anastomosis. It is used in the treatment or prevention of coccidiosis in both humans and animals.
Chemical properties
White to Off-White Solid
The Uses of Halofuginone hydrobromide
Halogenated derivative of Febrifugine. Halofuginone hydrobromide is used as an antiprotozoal (coccidiostat).
Definition
The hydrobromide salt of halofuginone, a semisynthetic quinazolinone alkaloid anticoccidial derived from the plant Dichroa febrifuga, with antifibrotic and potential antineoplastic activities. Halofuginone specifically inhibits collagen type I gene expression and matrix metalloproteinase 2 (MMP-2) gene expression, which may result in the suppression of angiogenesis, tumor stromal cell development, and tumor cell growth. These effects appear to be due to halofuginone-mediated inhibition of the collagen type I and MMP-2 promoters. Collagen type I and MMP-2 play important roles in fibro-proliferative diseases.
Biological Activity
Halofuginone hydrobromide is a high affinity competitive prolyl-tRNA synthetase inhibitor (Ki = 18.3 nM). Blocks expression of MMP2. Inhibits ECM invasion in vitro and lung metastasis by bladder cancer cells in mice. Inhibits the development of Th17-driven autoimmunity in a mouse model of multiple sclerosis by activating the amino acid response (AAR) pathway. Also antiparasitic.
Toxicology
Halofuginone HBr (Halofuginone hydrobromide) is toxic by inhalation, dermal and ocular routes and is very irritant to both the eye and the skin. It is considered also a skin sensitiser[1].
Storage
Store at -20°C
Mode of action
Halofuginone is an an ATP-dependent inhibitor prolyl-tRNA synthetase (Ki of 18.3 nM). Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. Blocks expression of MMP2 and inhibits ECM invasion in vitro and lung metastasis by bladder cancer cells in mice. Also inhibits the development of Th17-driven autoimmunity in a mouse model of multiple sclerosis by activating the amino acid response (AAR) pathway.
References
[1] Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, et al. “Safety of a feed additive consisting of halofuginone hydrobromide (STENOROL?) for chickens for fattening and turkeys (Huvepharma N.V.).” EFSA Journal 20 12 (2022).
Properties of Halofuginone hydrobromide
| Melting point: | 247° (dec) |
| storage temp. | Store at -20°C |
| solubility | Soluble to 100 mM in DMSO. |
| form | Solid |
| form | neat |
| color | White to off-white |
Safety information for Halofuginone hydrobromide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Halofuginone hydrobromide
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Halofuginone hydrobromide CAS 64924-67-0View Details
Halofuginone hydrobromide CAS 64924-67-0View Details
64924-67-0 -
 ProRS Inhibitor, Halofuginone CAS 64924-67-0View Details
ProRS Inhibitor, Halofuginone CAS 64924-67-0View Details
64924-67-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
