GSK621
Synonym(s):6-Chloro-5-(2′-hydroxy-3′-methoxy-[1,1′-biphenyl]-4-yl)-3-(3-methoxyphenyl)-1,5-dihydro-2H-pyrrolo[3,2-d]pyrimidine-2,4(3H)-dione;6-Chloro-5-(2′-hydroxy-3′-methoxy[1,1′-biphenyl]-4-yl)-3-(3-methoxyphenyl)-1H-pyrrolo[3,2-d]pyrimidine-2,4(3H,5H)-dione
- CAS NO.:1346607-05-3
- Empirical Formula: C26H20ClN3O5
- Molecular Weight: 489.91
- MDL number: MFCD28502280
- Update Date: 2026-01-13 11:17:14
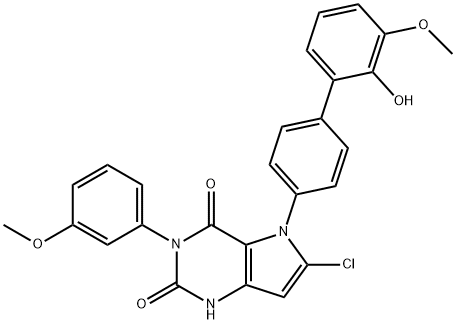
What is GSK621?
Biological Activity
gsk621 is a specific and potent agonist of amp-activated protein kinase (ampk) with ic50 values ranging from 13 to 30 μm for cell lines [1].ampk is a heterotrimeric serine/threonine kinase. it is a sensor of cellular energy. it regulates multiple cellular metabolic pathways. activation of ampk inactivates the phosphorylation of acetyl-coa carboxylase (acc), and hence inhibits the biosynthesis of fatty acid. activation of ampk also inhibits protein synthesis that is dependent on mammalian target of rapamycin complex 1. ampk also promotes autophagy, fatty acid oxidation, glucose uptake and glycolysis [1].in cells, at the opposite, gsk621 showed more potency to activate ampk than a-769662, based on the acc phosphorylation level. in molm-14 cells, 200 μm a-769662 is just as potent as 30 μm gsk621 to induce the phosphorylation of ulk1 (s555) and acc (s79), two direct ampk substrates. in aml cell lines (oci-aml3, hl-60 and molm-14) and primary aml samples, the phosphorylation at ampkα t172, a marker of ampk activation, was markedly increased, and the phosphorylation of ulk1 (s555) and acc (s79) was stimulated by gsk621 [1].in animals with xenograft molm-14 cells, intraperitoneal injection of gsk621 at 30 mg/kg twice daily reduced leukemia growth and markedly extended survival compared to treatment with 10 mg/kg gsk621 twice daily or vehicle. these results correlated to increased ampk activity indicated by the induction of apoptosis and increased acc s79 phosphorylation [1].
in vitro
GSK621 (30 μM) induces AMPKα T172, ACC (S79) and ULK1 (S555) phosphorylation.
It induces autophagy and apoptosis.
It treatment also induces PERK phosphorylation , a marker of ER stress, in AML cells.
Cell Proliferation Assay
| Cell Line: | MV4-11, OCI-AML3, OCI-AML2, HL-60, Kasumi, HEL, UT7, NB4, TF-1, KG1A, Nomo p28, SKM-1, U937 , YHP1, MOLM-14, Mo7e, K562, MOLM-13, EOL-1, SET-2 AML cell lines. 0-30 μM. |
| Concentration: | 0-30 μM. |
| Incubation Time: | 4 d. |
| Result: | IC 50 values ranged from 13 to 30 μM. Reduced the proliferation of all 20 lines and increased apoptosis in 17 (85%) lines. < br/> |
Cell Autophagy Assay. < /p>
| Cell Line: | AML cell lines and primary AML samples. | < /tr>
| Concentration: | 30 μM. |
| Incubation Time: | 24 h. |
| Result: | Induced the formation of numerous intracytoplasmic vacuoles including autophagosomes. |
in vivo
GSK621 (30 mg/kg, ip twice daily) exhibits significant anti-tumor activity in MOLM-14 cells xenograft.
| Animal Model: < /td> | MOLM-14 cells xenografted into nude mice. |
| Dosage: | 30 mg/kg. |
| Administration: | IP twice daily. |
| Result: | Reduced leukemia growth and significantly extended survival compared to vehicle-treated animals or those treated with 10 mg /kg twice daily. |
References
[1]. sujobert p, poulain l, paubelle e, et al. co-activation of ampk and mtorc1 induces cytotoxicity in acute myeloid leukemia. cell reports, 2015, 11(9): 1446-1457.
Properties of GSK621
| Density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥28.5 mg/mL in DMSO |
| form | crystalline solid |
| pka | 9.01±0.40(Predicted) |
| color | Off-white to light yellow |
Safety information for GSK621
Computed Descriptors for GSK621
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideYou may like
-
 GSK621 CAS 1346607-05-3View Details
GSK621 CAS 1346607-05-3View Details
1346607-05-3 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
