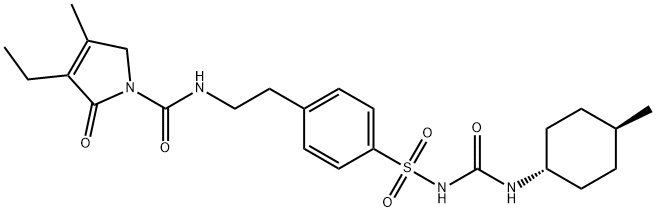
Glimepiride
Synonym(s):trans-3-Ethyl-2,5-dihydro-4-methyl-N-[N-[4-[[[[(4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxamide;Glimepiride;HOE-490
- CAS NO.:93479-97-1
- Empirical Formula: C24H34N4O5S
- Molecular Weight: 490.62
- MDL number: MFCD00878417
- EINECS: 642-919-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
What is Glimepiride?
Absorption
Glimepiride is completely absorbed after oral administration within 1 hour of administration with a linear pharmacokinetics profile. Following administration of a single oral dose of glimepiride in healthy subjects and with multiple oral doses with type 2 diabetes, the peak plasma concentrations (Cmax) were reached after 2 to 3 hours post-dose. Accumulation does not occur after multiple doses. When glimepiride was given with meals, the time to reach Cmax was increased by 12% while the mean and AUC (area under the curve) were decreased by 8 to 9%, respectively. In a pharmacokinetic study of Japanese patients with T2DM, Cmax value in once-daily dose was higher than those in twice-daily doses. The absolute bioavailability of glimepiride is reported to be complete following oral administration.
Toxicity
The oral LD50 value in rats is > 10000 mg/kg. The intraperitoneal LD50 value in rats is reported to be 3950 mg/kg . Although glimepiride is reported to have fewer risks of hypoglycemia compared to other sulfonylureas such as glyburide, overdosage of glimepiride may result in severe hypoglycemia with coma, seizure, or other neurological impairment may occur. This can be treated with glucagon or intravenous glucose. Continued observation and additional carbohydrate intake may be necessary since hypoglycemia may recur after apparent clinical recovery.
In a study of rats given doses of up to 5000 parts per million (ppm) in complete feed for 30 months, there were no signs of carcinogenesis. Meanwhile, the administration of glimepiride at a dose much higher than the maximum human recommended dose for 24 months in mice resulted in an increase in benign pancreatic adenoma formation in a dose-related manner, which was thought to be the result of chronic pancreatic stimulation. Glimepiride was non-mutagenic in in vitro and in vivo mutagenicity studies. In male and female rat studies, glimepiride was shown to have no effects on fertility.
Description
Glimepiride, the first of a new generation of sulfonylurea drugs, was introduced in Sweden in 1995 as a first-line therapy to lower blood glucose in patients with type II diabetes. Sulfonylureas exert their hypoglycemic function primarily by direct stimulation of insulin secretion in glucose-insensitive pancreatic β-cells and GLUT translocation in insulin-resistant fat and muscle cells. Once-daily, orally administered glimepiride in diabetes patients showed a more rapid and longer lasting glucose-lowering effect than the commonly used agent glibenclamide. Glimepiride can be used either as a monotherapy or in combination with insulin.
Chemical properties
White Cyrstalline Solid
Originator
Hoechst Marion Roussel (Germany)
The Uses of Glimepiride
Glimepiride induces the PI3 kinase (PI3K) and Akt pathway, along with insulin receptor substrate-1/2 and endothelial nitric oxide synthase. Glimepiride also increases osteoblast proliferation and differentiation, which is thought to be related to its ability to activate the PI3K and Akt pathway. Furthermore, Glimepiride enhances intrinsic peroxisome proliferator-activated receptor γ activity. Glimepiride also increases protein expression of glucose transports 1 and 4, and is a potent KIR channel blocker. Potent Kir6 (KATP) channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac Kir6 channels with an IC50 of 6.8 nM.
The Uses of Glimepiride
A sulfonylurea hypoglycemic agent. Used as an antidiabetic
The Uses of Glimepiride
anticonvulsant
The Uses of Glimepiride
For concomitant use with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus.
What are the applications of Application
Glimepiride is a third generation sulfonylurea compound that induces the PI3K and Akt pathway,
Background
First introduced in 1995, glimepiride is a member of the second-generation sulfonylurea (SU) drug class used for the management of type 2 diabetes mellitus (T2DM) to improve glycemic control. Type 2 diabetes is a metabolic disorder with increasing prevalences worldwide; it is characterized by insulin resistance in accordance with progressive β cell failure and long-term microvascular and macrovascular complications that lead to co-morbidities and mortalities. Sulfonylureas are one of the insulin secretagogues widely used for the management of type 2 diabetes to lower blood glucose levels. The main effect of SUs is thought to be effective when residual pancreatic β-cells are present, as they work by stimulating the release of insulin from the pancreatic beta cells and they are also thought to exert extra-pancreatic effects, such as increasing the insulin-mediated peripheral glucose uptake.
Glimepiride works by stimulating the secretion of insulin granules from pancreatic islet beta cells by blocking ATP-sensitive potassium channels (KATP channels) and causing depolarization of the beta cells. Compared to glipizide, another second SU drug, glimepiride has a longer duration of action. It is sometimes classified as a third-generation SU because it has larger substitutions than other second-generation SUs. Compared to other SUs, glimepiride was associated with a lower risk of developing hypoglycemia and weight gain in clinical trials as well as fewer cardiovascular effects than other SUs due to minimal effects on ischemic preconditioning of cardiac myocytes. It is effective in reducing fasting plasma glucose, postprandial glucose, and glycosylated hemoglobin levels and is considered to be a useful, cost-effective treatment option for managing type 2 diabetes mellitus. Glimepiride was approved by the Food and Drug Administration (FDA) in the United States in 1995 for the treatment of T2DM. It is commonly marketed under the brand name Amaryl as oral tablets and is typically administered once daily.
Indications
Glimepiride is indicated for the management of type 2 diabetes in adults as an adjunct to diet and exercise to improve glycemic control as monotherapy.
It may also be indicated for use in combination with metformin or insulin to lower blood glucose in patients with type 2 diabetes whose high blood sugar levels cannot be controlled by diet and exercise in conjunction with an oral hypoglycemic (a drug used to lower blood sugar levels) agent alone.
Definition
ChEBI: Glimepiride is a sulfonamide, a N-acylurea and a N-sulfonylurea. It has a role as a hypoglycemic agent and an insulin secretagogue.
Manufacturing Process
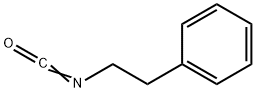
By heating of a mixture of 3-ethyl-4-methyl-2-pyrrolone and 2- phenylethylisocyanate at 150°C is obtained 3-ethyl-4-methyl-2-oxo-3- pyrroline-1-(N-2-phenylethyl)-carboxamide, melting point 106°-108°C. Then the carboxamide are introduced in portions at 30°C into chlorosulfonic acid, and agitated for 1 hour at 40°C. The sulfochloride (melting point 172-175°C), introduced into concentrated ammonia, and heated for 30 min on a steam bath. The mixture of sulfonamide obtained (melting point 180°-182°C), of acetone and K2CO3 are refluxed with agitation for 6 hours. Subsequently the cyclohexyl isocyanate are added dropwise, and agitation is continued for 6 hours at boiling temperature. After standing overnight, the product is filtered, the crystals obtained are treated with dilute hydrochloric acid, and again filtered. It is prepared N-(4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1- carboxamido)ethyl]benzenesulfonyl)-N'-cyclohexyl urea; melting point 185°- 187°C (from acetone) (Glimepiride).
brand name
Amaryl (Sanofi Aventis).
Therapeutic Function
Oral hypoglycemic
General Description
Glimepiride is 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans-4-methylcyclohexyl)amino]-carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxamide; thiscompound can also be named as the urea—see precedingdiscussion (Amaryl, generic). Combinations are availablewith rosiglitazone in the United States (Avandaryl tablets;mg glimepiride/mg rosiglitazone as maleate salt: 1/4,2/4, 4/4, 2/8, 4/8); and with pioglitazone (Duetact tablets;mg glimepiride/ mg pioglitazone as hydrochloride salt:2/30, 4/30).
General Description
Glimepiride, 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)urea (Amaryl), is very similarto glipizide with the exception of their heterocyclic rings.Instead of the pyrazine ring found in glipizide, glimepiridecontains a pyrrolidine system. It is metabolized primarilythrough oxidation of the alkyl side chain of the pyrrolidine,with a minor metabolic route involving acetylation of theamine.
Biological Activity
Potent K ATP channel blocker and anti-diabetic agent. Inhibits pinacidil-activated cardiac K ATP channels with an IC 50 of 6.8 nM.
Biochem/physiol Actions
Glimepiride is a potent blocker of cardiac KATP channels activated by pinacidil with an IC50 of 6.8 nM.
Pharmacokinetics
Glimepiride stimulates the secretion of insulin granules from the pancreatic beta cells and improves the sensitivity of peripheral tissues to insulin to increase peripheral glucose uptake, thus reducing plasma blood glucose levels and glycated hemoglobin (HbA1C) levels. A multi-center, randomized, placebo-controlled clinical trial evaluated the efficacy of glimepiride (1–8 mg) as monotherapy titrated over 10 weeks compared with placebo in T2DM subjects who were not controlled by diet alone. In this study, there was a reduction in fasting plasma glucose (FPG) by 46 mg/dL, post-prandial glucose (PPG) by 72 mg/dL, and HbA1c by 1.4% more than the placebo. In another randomized study comprising of patients with T2DM receiving either placebo or one of the three doses (1, 4, or 8 mg) of glimepiride during a 14-week study period, all glimepiride regimens significantly reduced FPG, PPG, and HbA1c values (P < 0.001) compared to placebo by the end of the study period. The 4- and 8-mg doses of glimepiride were more effective than the 1-mg dose; however, the 4-mg dose provided a nearly maximal antihyperglycemic effect.
Clinical Use
Non-insulin dependent diabetes mellitus
Veterinary Drugs and Treatments
Glimepiride may potentially be a useful adjunct in the treatment of non-insulin dependent diabetes mellitus (NIDDM) in cats. Its duration of action in humans allows it to be dosed once daily, which could be of benefit in cats. It may also have fewer side effects than glipizide in cats.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: effects enhanced by NSAIDs.
Antibacterials: effects enhanced by chloramphenicol,
sulphonamides, tetracyclines and trimethoprim;
effect reduced by rifamycins.
Anticoagulants: effect possibly enhanced by
coumarins; also possibly changes to INR.
Antifungals: concentration increased by fluconazole
and miconazole and possibly voriconazole.
Lipid-regulating drugs: possibly additive
hypoglycaemic effect with fibrates.
Sulfinpyrazone: enhanced effect of sulphonylureas.
Metabolism
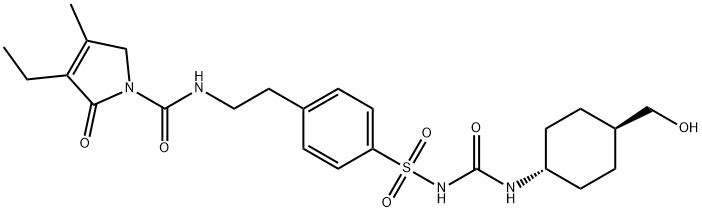
Glimepiride is reported to undergo hepatic metabolism. Following either an intravenous or oral dose, glimepiride undergoes oxidative biotransformation mediated by CYP2C9 enzyme to form a major metabolite, cyclohexyl hydroxymethyl derivative (M1), that is pharmacologically active. M1 can be further metabolized to the inactive metabolite carboxyl derivative (M2) by one or several cytosolic enzymes. M1 retained approximately one third of the pharmacologic activity of its parent in an animal model, with a half-life of 3-6 hours. However, whether the glucose-lowering effect of M1 is clinically significant is not clear.
Metabolism
The drug is extensively metabolised in the liver to two
main metabolites. The cytochrome P450 isoenzyme
CYP2C9 is involved in the formation of a hydroxy
derivative, which is further metabolised to a carboxy
derivative by cytosolic enzymes.
About 60% of a dose is eliminated in the urine and 40%
in the faeces.
Properties of Glimepiride
| Melting point: | 212.2-214.5 °C |
| Density | 1.29±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: >10 mg/mL |
| form | solid |
| pka | 5.10±0.10(Predicted) |
| color | white |
| Merck | 14,4440 |
| CAS DataBase Reference | 93479-97-1(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Pyrrole-1-carboxamide, 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[(trans- 4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo- (93479-97-1) |
Safety information for Glimepiride
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Glimepiride
Glimepiride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Glimepiride 98%View Details
Glimepiride 98%View Details -
 93479-97-1 98%View Details
93479-97-1 98%View Details
93479-97-1 -
 Glimepiride 98% (HPLC) CAS 93479-97-1View Details
Glimepiride 98% (HPLC) CAS 93479-97-1View Details
93479-97-1 -
 Glimepiride 99% (HPLC) CAS 93479-97-1View Details
Glimepiride 99% (HPLC) CAS 93479-97-1View Details
93479-97-1 -
 Glimepiride 95.00% CAS 93479-97-1View Details
Glimepiride 95.00% CAS 93479-97-1View Details
93479-97-1 -
 Glimepiride Powder API, Grade Standard: IPView Details
Glimepiride Powder API, Grade Standard: IPView Details
93479-97-1 -
 Glimepiride CAS NO. 93479-97-1View Details
Glimepiride CAS NO. 93479-97-1View Details
93479-97-1 -
 Glimepiride Ip APIView Details
Glimepiride Ip APIView Details
93479-97-1
