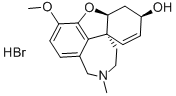
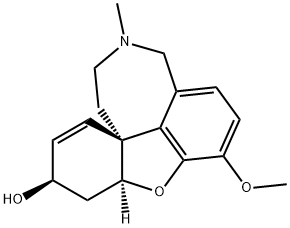
Galanthamine hydrobromide
Synonym(s):Galanthamine, Hydrobromide - CAS 69353-21-5 - Calbiochem;Nivalin, HBr
- CAS NO.:69353-21-5
- Empirical Formula: C17H22BrNO3
- Molecular Weight: 368.27
- MDL number: MFCD00067672
- EINECS: 217-780-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 13:16:57
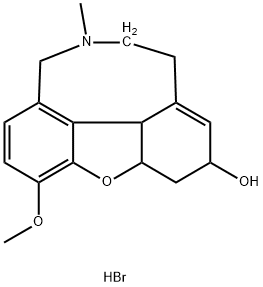
What is Galanthamine hydrobromide?
General Description
A competitive and reversible inhibitor of acetylcholinesterase. Antimyasthenic agent. Can partially reverse the effects of scopolamine-induced amnesia in rats. Reported to improve learning and short-term memory in animal models.
Biochem/physiol Actions
Primary TargetAcetylcholinesterase
Clinical Use
Galantamine, which was introduced in 2001, is an alkaloid found in plants of the family Amaryllidaceae, which includes the daffodil (Narcissus pseudonarcissus) and snowflake (Leucojum aestivum). It is a reversible inhibitor of AChE, but it does not appear to inhibit butyrylcholinesterase. Because it is a tertiary amine and can cross the blood-brain barrier, it is indicated for treatment of mild-to-moderate AD and dementia. It has been used outside the U.S. for more than 30 years as an anticurare agent in anesthesia. Galantamine differs from other cholinesterase inhibitors, because it allosterically binds to nicotinic receptors, giving it a dual cholinergic action.
Metabolism
It is metabolized (75%) by CYP2D6 and CYP3A4 to afford the normethyl, O-desmethyl, and O-desmethylnormethyl metabolites, along with some other minor metabolites. Unlike tacrine, galantamine is not associated with hepatotoxicity. Its elimination half-life is 5.7 hours.
Properties of Galanthamine hydrobromide
| storage temp. | Sealed in dry,Room Temperature |
| form | White solid |
Safety information for Galanthamine hydrobromide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Galanthamine hydrobromide
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Galanthamine, Hydrobromide CAS 69353-21-5View Details
Galanthamine, Hydrobromide CAS 69353-21-5View Details
69353-21-5 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8