GADOBUTROL
- CAS NO.:770691-21-9
- Empirical Formula: C18H30GdN4O9
- Molecular Weight: 603.71
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:26:48
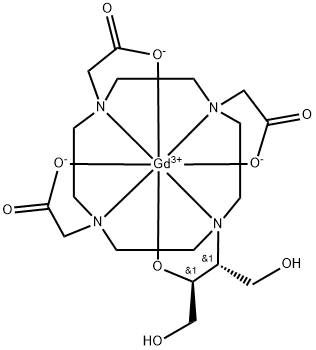
What is GADOBUTROL?
Absorption
With normal renal function, the AUC is 1.1 ± 0.1 mmol·h/L.
Toxicity
Lethality was observed in rodents after a single intravenous administration of 20 mmol/kg. This represents a dose of at least 2 orders of magnitude
higher than the standard single diagnostic dose in humans (0.1 mmol/kg).
No carcinogenicity studies have been conducted.
No mutagenesis was observed in vitro in reverse mutation tests in bacteria, or in the HGPRT (hypoxanthine-guanine phosphoribosyl transferase) test using Chinese hamster V79 cells. Similarly, no mutagenesis was seen in chromosome abberation tests of human peripheral blood lymphocytes. It was also negative in in-vivo micronucleus tests in mice following a 0.5mmol/kg intravenous injection.
No fertility or reproductive impairment was observed in male and female rates given doses 12.2 times human equivalent doses, based on body surface area.
Intolerance reactions local to the injection site have been observed in rabbits after paravenous administration, and are associated with the infiltration of inflammatory cells, suggesting the possibility of local irritation if the contrast medium leaks around veins in a clinical setting.
The Uses of GADOBUTROL
Gadobutrol is a gadolinium-based MRI contrast agent (GBCA).
Background
Intravenous gadobutrol is a second-generation extracellular non-ionic macrocyclic GBCA (gadolinium-based contrast agent) used in magnetic resonance imaging (MRI) in adults and children older than 2 years of age. It may help visualize and detect vascular abnormalities in the blood brain barrier (BBB) and central nervous system (CNS).
In patients with impaired renal function, gadolinium based contrast agents increase the risk of nephrogenic systemic fibrosis (NSF). A physician should be contacted if symptoms of NSF are encountered, such as dark or red patches on the skin; stiffness in joints; trouble moving, bending or straightening arms, hands, legs or feet; burning, itching, swelling, scaling, hardening and tightening of skin; pain in hip bones or ribs; or muscle weakness.
Common adverse reactions that may be experienced include headache, nausea, feeling hot, abnormal taste, and warmth, burning or pain local to the injection site.
General precautions should be taken in patients who are pregnant or breastfeeding, or who have a history of allergic reaction to contrast media, bronchial asthma or an allergic respiratory disorder.
Indications
Gadobutrol is indicated for use with magnetic resonance imaging for the following diagnostic processes:
Pharmacokinetics
Even at low concentrations Gadobutrol can lead to distinct shortening of relaxation times of protons in plasma. At physiological conditions (pH=7, temperature=37°C), and 1.5T, the relaxivity (r1) is 5.2L/(mmol·sec) based on the relaxation times (T1), while the relativity (r2) is 6.1L/(mmol·sec) based on relaxation times (T2).
Magnetic field strength has only slight influence on relaxivities.
Drug concentration and r1 relaxivity may contribute to a T1 shortening effect, which may improve tissue visualization.
Metabolism
Gadobutrol is not metabolized.
Properties of GADOBUTROL
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Water (Slightly) |
| form | Solid |
| color | White to Off-White |
Safety information for GADOBUTROL
Computed Descriptors for GADOBUTROL
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 770691-21-9 Gadobutrol 99%View Details
770691-21-9 Gadobutrol 99%View Details
770691-21-9 -
 Gallium 10-(1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-tricarboxylate 95% CAS 770691-21-9View Details
Gallium 10-(1,3,4-trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4,7-tricarboxylate 95% CAS 770691-21-9View Details
770691-21-9 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8