Fruquintinib
- CAS NO.:1194506-26-7
- Empirical Formula: C21H19N3O5
- Molecular Weight: 393.39
- MDL number: MFCD28502149
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
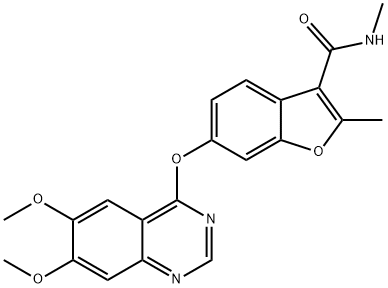
What is Fruquintinib?
Description
Fruquintinib is a VEGFR inhibitor (IC50s = 33, 35, and 0.5 nM for VEGFR1, -2, and -3, respectively). It also inhibits RET, FGFR1, and c-Kit (IC50s = 128, 181, and 458 nM, respectively) in a panel of 253 kinases. Fruquintinib inhibits VEGF-A-induced proliferation of human umbilical vein endothelial cells (HUVECs) and VEGF-C-induced proliferation of human lymphatic endothelial cells (HLECs; IC50s = 1.7 and 4.2 nM, respectively). It decreases tube formation by HUVECs by 74 and 94% when used at concentrations of 30 and 300 nM, respectively. Fruquintinib (0.5-20 mg/kg per day for 21 days) reduces tumor growth in BGC-823, HT-29, Caki-1, and NCI H460 mouse xenograft models.
The Uses of Fruquintinib
Fruquintinib is a multi-targeted tyrosine kinase inhibitor, a third-line regimen involved in the treatment of advanced non-small cell lung cancer in humans. Fruquintinib is a potent, highly selective and orally active inhibitor of VEGFR1, 2, 3 tyrosine kinases.
The Uses of Fruquintinib
Fruquintinib (HMPL-013) is a highly selective VEGFR-1/2/3 tyrosine kinase inhibitor. It was approved by the FDA on 8 November 2023 for the treatment of adult patients with metastatic colorectal cancer. and these patients had previously received fluoropyrimidine, oxaliplatin and irinotecan chemotherapy, anti-vascular endothelial growth factor therapy, and anti-epidermal growth factor receptor (EGFR) therapy. Fruquintinib has also been shown to improve cognitive deficits and pathological changes in a mouse model of cerebral amyloid angiopathy (CAA).
Mechanism of action
By blocking the vascular endothelial growth factor signalling pathway, Fruquintinib inhibits the formation of new blood vessels in tumours, thereby reducing blood supply and inhibiting tumour growth.
in vitro
fruquintinib was found to inhibit vegfr2 with an ic50 of 25 nmol/l. the kinase selectivity of fruquintinib was evaluated against a panel of 253 kinases. the results showed that fruquintinib inhibited vegfr family members with weak inhibition of ret, fgfr-1 and c-kit kinases [1].
in vivo
anti-tumor activity of fruquintinib was evaluated in a variety of tumor xenografts. the results from gastric cancer bgc-823 model seemed to indicate that the drug concentration needs to be at least maintained above ec85 for around 8 hours in order to achieve >80% tumor growth inhibition. bgc-823 was found to be most sensitive to fruquintinib [1].
References
1) Sun?et al.?(2014)?Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1,2,3 tyrosine kinases for cancer therapy; Cancer Biol. Ther.?15?1635 2) Li?et al.?(2018)?Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial; JAMA?319?2486 3) Lu?et al.?(2018)?Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Fruquintinib After Two Prior Chemotherapy Regimens in Chinese Patients With Advanced Nonsquamous Non-Small-cell Lung Cancer; J. Clin. Oncol.?36?1207
Properties of Fruquintinib
| Boiling point: | 600.5±55.0 °C(Predicted) |
| Density | 1.302±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 5 mg/ml). |
| form | solid |
| pka | 14.35±0.46(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
| InChI | InChI=1S/C21H19N3O5/c1-11-19(20(25)22-2)13-6-5-12(7-16(13)28-11)29-21-14-8-17(26-3)18(27-4)9-15(14)23-10-24-21/h5-10H,1-4H3,(H,22,25) |
Safety information for Fruquintinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fruquintinib
| InChIKey | BALLNEJQLSTPIO-UHFFFAOYSA-N |
| SMILES | O1C2=CC(OC3=C4C(=NC=N3)C=C(OC)C(OC)=C4)=CC=C2C(C(NC)=O)=C1C |
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 1194506-26-7 Fruquintinib 98%View Details
1194506-26-7 Fruquintinib 98%View Details
1194506-26-7 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
