Fosnetupitant
- CAS NO.:1703748-89-3
- Empirical Formula: C31H35F6N4O5P
- Molecular Weight: 688.61
- Update Date: 2026-01-05 11:31:08
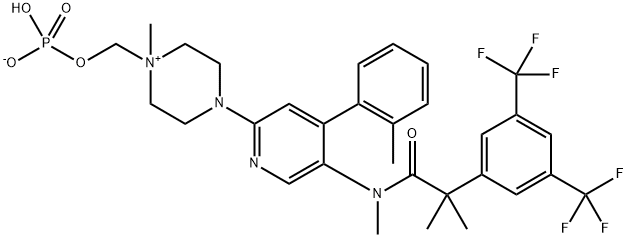
What is Fosnetupitant?
Absorption
Following single intravenous doses of Akynzeo for injection in patients (235 mg fosnetupitant and 0.25 mg palonosetron infused in 30 minutes) or fosnetupitant in healthy subjects (235 mg fosnetupitant infused in 30 minutes), maximum concentration of fosnetupitant was achieved at the end of the 30-minute infusion .
Oral bioavailability in each species varied substantially between animals, with 42-105%, 34-83% and 37-62% in rats, dogs, and monkeys. The large variation is most likely due to the low numbers of animals used in the studies .
Toxicity
Most common adverse reactions (≥3%) for AKYNZEO capsules are headache, asthenia, dyspepsia, fatigue, constipation and erythema .
The safety profile of Akynzeo for injection is generally similar to that seen with Aynzeo capsules , .
Currently a repeated dose safety study is ongoing in patients receiving anthracycline plus cyclophosphamide to further establish the safety profile in this setting .
The Uses of Fosnetupitant
Fosnetupitant is an anti-emetic used in the treatment of radio-and-chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy.
Background
In April 2018, the U.S. Food and Drug Administration (FDA) and the Swiss company Helsinn approved the intravenous formulation of AKYNZEO? (NEPA, a fixed antiemetic combination of fosnetupitant, 235mg, and palonosetron, 0.25mg) as an alternative treatment option for patients experiencing chemotherapy-induced nausea and vomiting . Fosnetupitant is the pro-drug form of netupitant .
Generally, 25% to 30% of patients with a diagnosis of cancer receive chemotherapy as a treatment modality and 70% to 80% of these patients undergoing chemotherapy treatment may experience nausea and vomiting as major side effects. Considered one of the most distressing side effects of chemotherapy, nausea and vomiting has an immense impact on the quality of life of patients receiving certain antineoplastic therapies .
Indications
Indicated in combination palonosetron (as the drug Akynzeo) and dexamethasone in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy .
The following are indications listed on the EMA label :
Prevention of acute and delayed nausea and vomiting associated with highly emetogenic cisplatin-based cancer chemotherapy .
Prevention of acute and delayed nausea and vomiting associated with moderately emetogenic cancer chemotherapy .
Pharmacokinetics
In the combination drug, Akynzeo, palonosetron prevents nausea and vomiting during the acute phase and fosnetupitant prevents nausea and vomiting during both the acute and delayed phase after cancer chemotherapy .
Neurokinin-1 (NK-1) inhibitor drugs, such as netupitant, possess unique anxiolytic, antidepressant, and antiemetic properties .
Metabolism
Fosnetupitant is the prodrug of netupitant .
Netupitant is a moderate inhibitor and substrate of CYP3A4 .
Akynzeo should be used with caution in patients receiving concomitant medications that are primarily metabolized through CYP3A4 systems. One dose of netupitant 300 mg significantly inhibits CYP3A4 for about 6 days. It is avisable to avoid concomitant use of drugs that are CYP3A4 substrates for one week. If not possible, consider dose reduction of CYP3A4 substrates .
In the human, rat, dog, minipig and marmoset liver microsomal incubations, two major metabolites, an N-demethylation product (M1) _and an _N-oxidation product (M2), in addition to hydroxylation products (M3), were identified in all species. CYP3A4 was found to be responsible for the oxidation of netupitant to the same metabolites observed also in the incubations with human liver microsomes. Metabolism was extensive, with the metabolites generally achieving greater concentrations than parent drug witin 24 hours. M1 and M2 exposure was similar in rat to humans, but higher in dogs, however M3 was lower in both species than in humans .
Properties of Fosnetupitant
| Melting point: | >140°C (dec.) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Fosnetupitant
Computed Descriptors for Fosnetupitant
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 1703748-89-3 Fosnetupitant 98%View Details
1703748-89-3 Fosnetupitant 98%View Details
1703748-89-3 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
