Fenbufen
Synonym(s):γ-Oxo-(1,1′-biphenyl)-4-butanoic acid
- CAS NO.:36330-85-5
- Empirical Formula: C16H14O3
- Molecular Weight: 254.29
- MDL number: MFCD00056701
- EINECS: 252-979-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
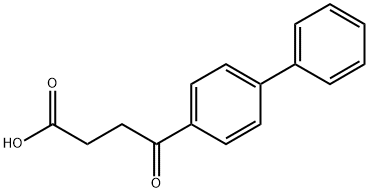
What is Fenbufen?
Description
Fenbufen has been found to be an effective, well-tolerated drug for the treatment of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis. Fenbufen is prepared by the Friedel-Crafts (aluminum chloride – nitrobenzene) acylation of biphenyl with succinic anhydride. The compound is metabolized in humans first to 4-hydroxy-4-biphenylbutyric acid (tmax 2.5 h) then to 4-biphenyl acetic acid (tmax 7.5 h). Both metabolites are more active than fenbufen itself and circulate for several hours (t1/2 10 h). This slow conversion of fenbufen to active metabolites having relatively long plasma half-lives allows for once a day dosing with this agent.
Chemical properties
White Solid
Originator
Cinopal,Cyanamid,Italy,1976
The Uses of Fenbufen
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory
The Uses of Fenbufen
muscle relaxant (smooth)
Background
Fenbufen is a non-steroidal anti-inflammatory drug used primarily to treat inflammation in osteoarthritis, ankylosing spondylitis, and tendinitis. It can also be used to relieve backaches, sprains, and fractures. Fenbufen is available as a capsule or tablet sold with the brand names Cepal, Cinopal, Cybufen, Lederfen, and Reugast. Fenbufen acts by preventing cyclooxygenase from producing prostaglandins which can cause inflammation.
What are the applications of Application
Fenbufen is an anti-inflammatory analgesic
Definition
ChEBI: Fenbufen is a member of biphenyls and a 4-oxo monocarboxylic acid. It has a role as a non-steroidal anti-inflammatory drug.
Manufacturing Process
135 g of aluminum chloride is dissolved in 500 ml of nitrobenzene, the solution being held below 10°C by external cooling. A finely ground mixture of 50 g of succinic anhydride and 75 g of biphenyl is added to the stirred solution, the temperature being held below 10°C. It is then held at room temperature for four days. After pouring the reaction mixture into a solution of 150 ml of concentrated hydrochloric acid in 1 liter of ice water, the nitrobenzene is removed by steam distillation. The solid is collected, dissolved in 4 liters of 3% hot sodium carbonate solution, clarified, and reprecipitated by the addition of excess 6N sulfuric acid solution. The crude product is collected, dried, and recrystallized from ethanol to give the pure subject compound, MP 185°C to 187°C.
Therapeutic Function
Antiinflammatory
General Description
Fenbufen belongs to the class of non-steroidal anti-inflammatory drugs, widely used as an antipyretic and analgesic in medical applications. Its mode of action involves the inhibition of cyclooxygenase enzyme and thereby prevents the synthesis of certain prostaglandins.
Trade name
Clincopal (Lederle, Spain), Lederfen (Lederle, UK), Napanol (Lederle, Japan).
Safety Profile
Poison by ingestion,intraperitoneal, and subcutaneous routes. Human systemiceffects by ingestion: cough, sweating, body temperature.An experimental teratogen. Other experimentalreproductive effects. An anti-inflammatory agent. Whenheated to decomposi
Metabolism
Not Available
Properties of Fenbufen
| Melting point: | 184-187 °C (lit.) |
| Boiling point: | 357.5°C (rough estimate) |
| Density | 1.1565 (rough estimate) |
| refractive index | 1.5600 (estimate) |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble in water, slightly soluble in acetone, in ethanol (96 per cent) and in methylene chloride. |
| form | neat |
| pka | pKa 4.3(H2O tunde?ned Iunde?ned) (Uncertain) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 2.212mg/L(25 ºC) |
| Merck | 13,3990 |
| EPA Substance Registry System | [1,1'-Biphenyl]-4-butanoic acid, .gamma.-oxo- (36330-85-5) |
Safety information for Fenbufen
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Fenbufen
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Fenbufen 95% CAS 36330-85-5View Details
Fenbufen 95% CAS 36330-85-5View Details
36330-85-5 -
 Fenbufen >98% (HPLC) CAS 36330-85-5View Details
Fenbufen >98% (HPLC) CAS 36330-85-5View Details
36330-85-5 -
 Fenbufen CAS 36330-85-5View Details
Fenbufen CAS 36330-85-5View Details
36330-85-5 -
 Fenbufen CAS 36330-85-5View Details
Fenbufen CAS 36330-85-5View Details
36330-85-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
