Chymotrypsin Substrate II, Fluorogenic
Synonym(s):Chymotrypsin Substrate II, Fluorogenic - CAS 88467-45-2 - Calbiochem;Suc-AAPF-AMC
- CAS NO.:88467-45-2
- Empirical Formula: C34H39N5O9
- Molecular Weight: 661.7
- MDL number: MFCD00080246
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:12
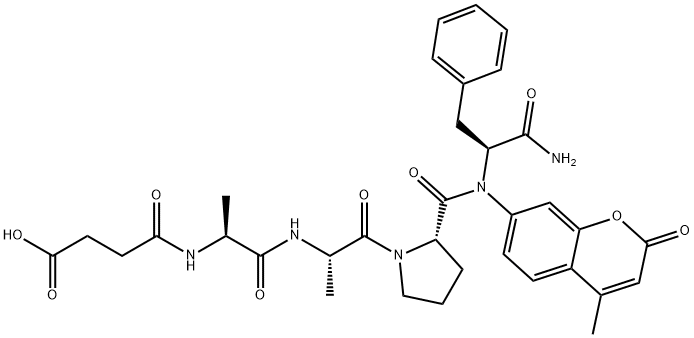
What is Chymotrypsin Substrate II, Fluorogenic?
Description
Chymotrypsin Substrate II, Fluorogenic finds widespread utility across diverse scientific research applications, encompassing the exploration of protein-protein interactions, enzyme kinetics, and receptor binding. Notably, its cost-effectiveness and user-friendly nature render it an appealing choice for researchers. Additionally, this peptide has been instrumental in delving into the intricate world of cell signaling pathways and unraveling the mechanisms governing gene expression regulation. It is postulated to function as a substrate for enzymes while also engaging with receptors. The prevailing belief suggests that the peptide effectively binds to specific receptors, subsequently triggering their activation and instigating consequential alterations in cellular signaling pathways.
Chemical properties
White powder
The Uses of Chymotrypsin Substrate II, Fluorogenic
Fluorogenic substrate for chymotrypsin.
The Uses of Chymotrypsin Substrate II, Fluorogenic
Chymotrypsin Substrate II, Fluorogenic (cas# 88467-45-2) is a useful compound in organic synthesis and acts as a substrate for chymotrypsin enzyme.
Biological Activity
Sensitive fluorogenic substrate for the quantitative determination of chymotrypsin activity (excitation maximum: ~380 nm; emission maximum: ~460 nm).
References
[1] G B IRVINE C H W M Ennis. Visual detection of peptidase activity using fluorogenic substrates in a microtiter plate assay.[J]. Analytical biochemistry, 1990, 185 2: 304-307. DOI:10.1016/0003-2697(90)90298-n.
[2] LASZLO GRAF. Selective alteration of substrate specificity by replacement of aspartic acid-189 with lysine in the binding pocket of trypsin[J]. Biochemistry Biochemistry, 1987, 26 9: 2616-2623. DOI:10.1021/bi00383a031.
[3] OSHIMA G. Interaction of alpha-chymotrypsin with dimyristoyl phosphatidylcholine vesicles.[J]. Journal of biochemistry, 1984, 95 4: 1131-1136. DOI:10.1093/oxfordjournals.jbchem.a134701.
[4] OSHIMA G. Stimulation by phospholipid vesicles of proteolysis of egg white lysozyme by chymotrypsin.[J]. Journal of biochemistry, 1983, 5 1: 1615-1620. DOI:10.1093/OXFORDJOURNALS.JBCHEM.A134509.
Properties of Chymotrypsin Substrate II, Fluorogenic
| Boiling point: | 1093.0±65.0 °C(Predicted) |
| Density | 1.347±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Clear, colorless solution (10 mg/mL in Methanol) |
| form | White lyophilized solid |
| pka | 4.69±0.10(Predicted) |
| color | White to off-white |
Safety information for Chymotrypsin Substrate II, Fluorogenic
Computed Descriptors for Chymotrypsin Substrate II, Fluorogenic
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


You may like
-
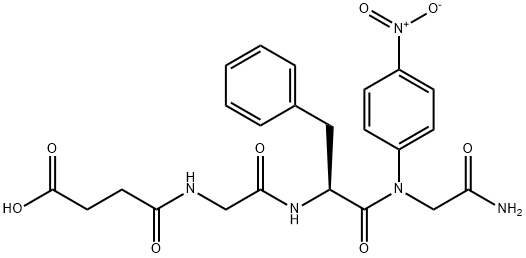
 Chymotrypsin Substrate II, Fluorogenic CAS 88467-45-2View Details
Chymotrypsin Substrate II, Fluorogenic CAS 88467-45-2View Details
88467-45-2 -
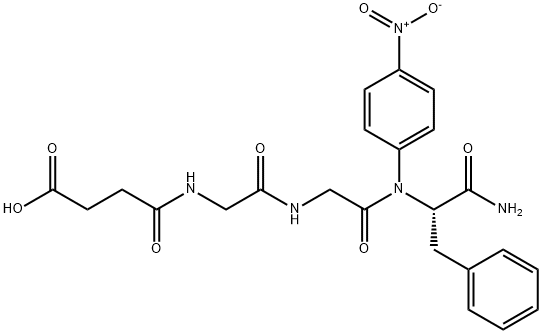
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
