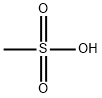
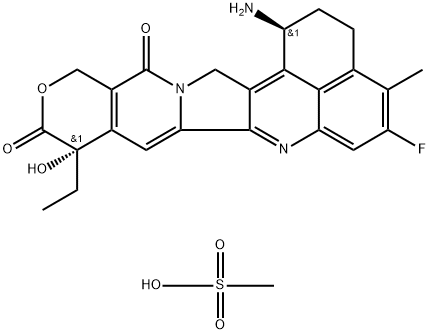
Exatecan mesylate
- CAS NO.:169869-90-3
- Empirical Formula: C25H26FN3O7S
- Molecular Weight: 531.56
- MDL number: MFCD04113012
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-21 16:49:52
What is Exatecan mesylate?
Description
Exatecan mesylate is a semisynthetic, water-soluble derivative of camptothecin with antineoplastic activity. Exatecan mesylate inhibits topoisomerase I activity by stabilizing the cleavable complex between topoisomerase I and DNA and inhibiting the religation of DNA breaks, thereby inhibiting DNA replication and triggering apoptotic cell death. This agent does not require enzymatic activation and exhibits greater potency than camptothecin and other camptothecin analogs.
The Uses of Exatecan mesylate
Exatecan Mesylate (DX8951f) is a topoisomerase I inhibitor with an IC50 value of 2.2 μM (0.975 μg/mL) for cancer research.
Synthesis
The synthesis of Exatecan mesilate is as follows:
500mL reaction flask,To a suspension of compound II (15 g, 1.0 eq) in ethylene glycol monomethyl ether (150 mL),After adding methanesulfonic acid (75 mL) and water (75 mL) under cooling,The solution was heated under reflux for 24 hours,The reaction was complete as detected by LCMS and cooled to 25°C.Remove the solvent under reduced pressure, add 2L methanol, stir for 2 hours and filter,The filter cake was washed with 200 mL methanol,Dry to obtain 9g of yellow ixitecan mesylate crude product,Yield 53.9%, HPLC purity: 96%.
Clinical claims and research
Exatecan mesylate is a synthetic camptothecin analog that is a more potent inhibitor of topoisomerase I than camptothecin, topotecan, and the active metabolite of irinotecan (CPT-11), SN-38. In preclinical studies, exatecan mesylate demonstrated broad antitumor activity compared with available camptothecin analogs. Furthermore, the antitumor activity observed in Phase I trials made exatecan mesylate an attractive compound for clinical development. A Phase II trial was designed to further evaluate the activity and pharmacokinetic (PK) profile of exatecan mesylate and determine its toxicity and tolerability in patients with metastatic breast carcinoma treated previously with anthracyclines and taxanes[1-2].
References
[1] M.D., Francisco J. Esteva and Ph.D. “A Phase II study of intravenous exatecan mesylate (DX-8951f) administered daily for 5 days every 3 weeks to patients with metastatic breast carcinoma.” Cancer 98 5 (2003): 900–907.
[2] D. Talbot. “Phase II study of Exatecan Mesylate (DX-8951f) in advanced NSCLC.” Lung Cancer 29 1 (2000): 55.
Properties of Exatecan mesylate
| storage temp. | 4°C, protect from light, stored under nitrogen |
| solubility | DMSO:7.41(Max Conc. mg/mL);13.94(Max Conc. mM) Water:0.0(Max Conc. mg/mL);0.19(Max Conc. mM) |
| form | A solid |
| color | Light yellow to green yellow |
| Stability: | Hygroscopic |
Safety information for Exatecan mesylate
Computed Descriptors for Exatecan mesylate
| InChIKey | BICYDYDJHSBMFS-OVCYJQQENA-N |
| SMILES | S(O)(=O)(=O)C.N[C@H]1CCC2C(C)=C(F)C=C3N=C4C5=CC6[C@@](C(=O)OCC=6C(=O)N5CC4=C1C3=2)(O)CC |&1:6,21,r| |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Exatecan mesylate CAS 169869-90-3View Details
Exatecan mesylate CAS 169869-90-3View Details
169869-90-3 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8