Ethylenediaminetetraacetic acid
Synonym(s):EDTA;Ethylenediaminetetraacetic acid;Ethylenedinitrilotetraacetic acid;EDTA solution;(Ethylenedinitrilo)tetraacetic acid
- CAS NO.:60-00-4
- Empirical Formula: C10H16N2O8
- Molecular Weight: 292.24
- MDL number: MFCD00003541
- EINECS: 200-449-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-15 13:03:24
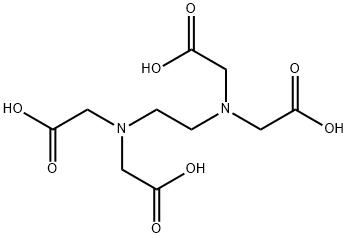
What is Ethylenediaminetetraacetic acid?
Absorption
Poorly absorbed from the gastrointestinal tract. Well absorbed following intramuscular injection.
Toxicity
Inadvertent administration of 5 times the recommended dose, infused intravenously over a 24 hour period, to an asymptomatic 16 month old patient with a blood lead content of 56 mcg/dl did not cause any ill effects. Edetate calcium disodium can aggravate the symptoms of severe lead poisoning, therefore, most toxic effects (cerebral edema, renal tubular necrosis) appear to be associated with lead poisoning. Because of cerebral edema, a therapeutic dose may be lethal to an adult or a pediatric patient with lead encephalopathy. Higher dosage of edetate calcium disodium may produce a more severe zinc deficiency.
Description
Ethylenediaminetetraacetic Acid (EDTA) is a common polydentate ligand. In EDTA, the hydrogen atoms are easily removed
in solution to produce anionic EDTA4-. In its anionic form Ethylenediaminetetraacetic Acid (EDTA) has six binding atoms, two
nitrogen and four oxygen.
Ethylenediaminetetraacetic Acid (EDTA) binds to a metal ion at the six binding sites, wrapping itself around the metal ion,
forming a very stable complex.the strong grasp of Ethylenediaminetetraacetic Acid (EDTA) on the metal ion is analogous
to a crab or lobster clamping down on an object with its claw, hence the name chelation.
Chemical properties
white crystals or powder
Chemical properties
Ethylenediaminetetraacetic acid is a solid.
History
Ethylenediaminetetraacetic Acid (EDTA) was first synthesized in the early 1930s by the German chemist Ferdinand Münz working for I. G. Farben. Münz, who was looking for a substitute for citric acid to use with dye solutions in the textile industry, was the first to patent a process for Ethylenediaminetetraacetic Acid (EDTA) synthesis in Germany in 1935. Münz subsequently applied for United States patents in 1936 and 1937 (U.S. Patent Number 2130505); his method involved reacting monochloroacetic acid (C2H3ClO2) and ethylene diamine (C2H8N2). Concurrent with Münz’s work, Frederick C. Bersworth in the United States synthesized Ethylenediaminetetraacetic Acid (EDTA) using different methods that gave greater yields and made EDTA’s commercial production economically viable. Bersworth syntheses involved reacting formaldehyde, amines, and hydrogen cyanide. Bersworth and Münz obtained patents for Ethylenediaminetetraacetic Acid (EDTA) production in the 1940s (U.S. Patent Numbers 2407645 and 2461519).
The Uses of Ethylenediaminetetraacetic acid
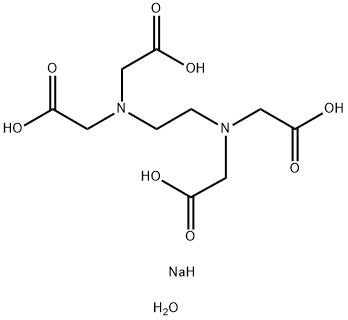
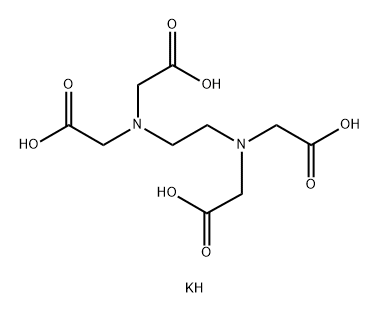
Ethylenediaminetetraacetic Acid (EDTA) is marketed in its salt forms such as sodium Ethylenediaminetetraacetic Acid (EDTA) or calcium EDTA. Ethylenediaminetetraacetic Acid (EDTA) hasindustrial and medical uses as a chelating agent. Much of its utility is related to the fact that metals and metal compounds are important catalysts in numerous reactions.
By chelatingmetals, Ethylenediaminetetraacetic Acid (EDTA) prevents the metal from catalyzing reactions, thereby limiting degradation, oxidation,and other undesirable reactions.the major industries using Ethylenediaminetetraacetic Acid (EDTA) and other chelatingagents are paper and pulp, cleaning products, chemicals, agriculture, and water treatment.
Ethylenediaminetetraacetic Acid (EDTA) is used with foods to preserve color and preserve flavor,prevent odors, maintain nutrient content, and extend shelf life. When used in beverages,Ethylenediaminetetraacetic Acid (EDTA) preserves color and stabilizes other ingredients such as citric acid and benzoates.
The Uses of Ethylenediaminetetraacetic acid
antispasmodic
The Uses of Ethylenediaminetetraacetic acid
Ethylenediamine-N,N,N’N’tetraacetic Acid (EDTA) is a powerful chelating agent; EDTA forms stable complexes with most metal ions. EDTA is used in treatment of lead and heavy metal poisoning of farm a nimals.
The Uses of Ethylenediaminetetraacetic acid
EDTA is helps boost a formulation’s preservative system and is also a chelating agent.
Background
A chelating agent, employed in pharmaceutical manufacturing and as a food additive, effectively sequesters various polyvalent cations. This versatile compound plays a crucial role in pharmaceutical processes, ensuring product stability and quality. Additionally, its application as a food additive underscores its significance in food preservation and enhancement, where it acts as a sequestrant, binding undesirable metal ions and preventing their adverse effects on food quality and shelf life.
Indications
For the reduction of blood levels and depot stores of lead in lead poisoning (acute and chronic) and lead encephalopathy, in both pediatric populations and adults.
Definition
An organic chelating agent.
Production Methods
Edetic acid may be prepared by the condensation of ethylenediamine
with sodium monochloroacetate in the presence of sodium
carbonate. An aqueous solution of the reactants is heated to about
90°C for 10 hours, then cooled, and hydrochloric acid is added to
precipitate the edetic acid.
Edetic acid may also be prepared by the reaction of ethylenediamine
with hydrogen cyanide and formaldehyde with subsequent
hydrolysis of the tetranitrile, or under alkaline conditions with
continuous extraction of ammonia.
Definition
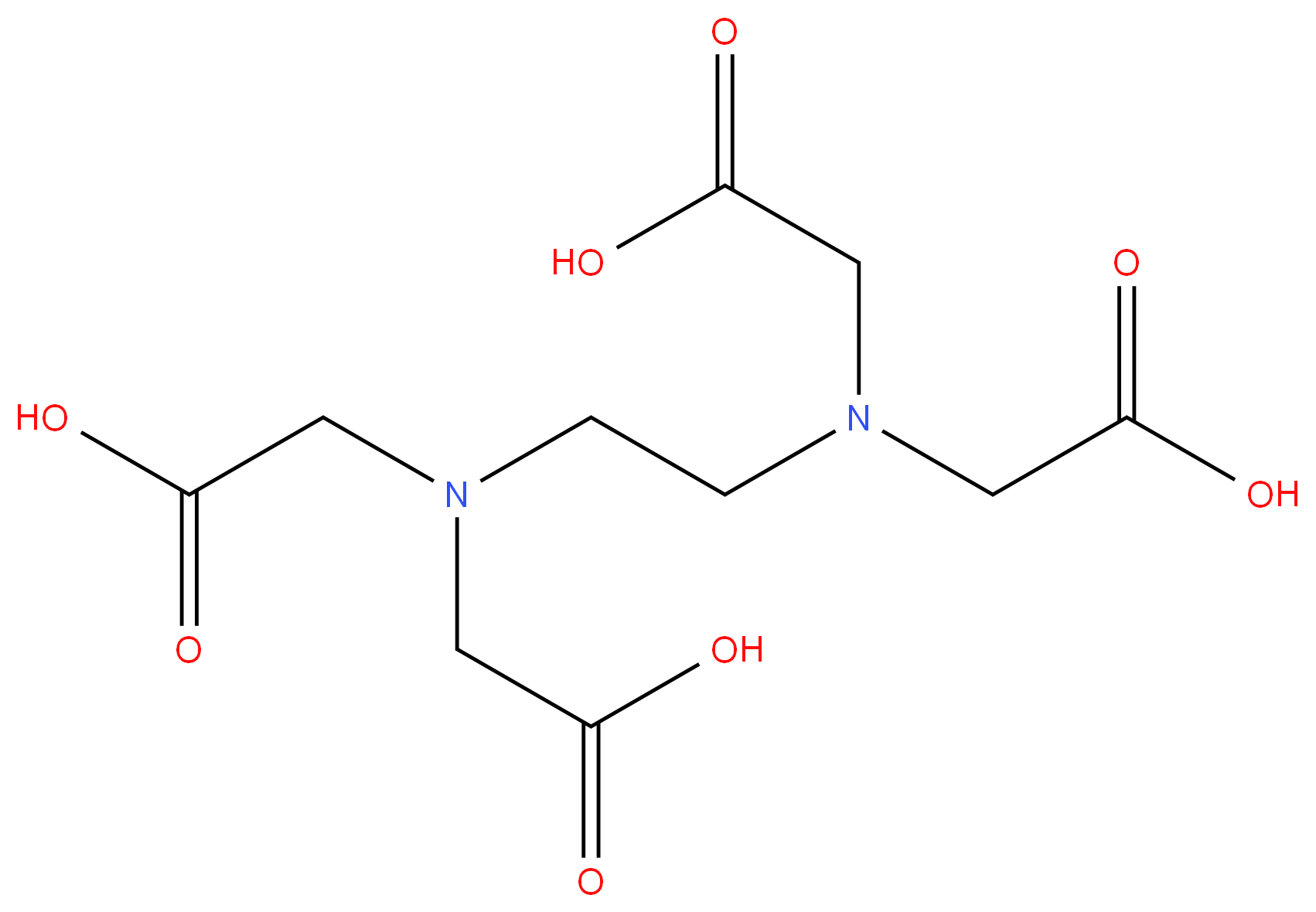
A compound with the formula (HOOCCH2)2N(CH2)2N(CH2COOH)2 It is used in forming chelates of transition metals.
brand name
Versene Acid (Dow Chemical).
General Description
Ethylenediamine tetraacetic acid is a colorless crystalline solid. Ethylenediaminetetraacetic acid is slightly soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ethylenediaminetetraacetic acid is used in chemical analysis, to make detergents and cleaning compounds, and for many other uses.
Air & Water Reactions
Slightly soluble in water.
Agricultural Uses
EDTA is short for ethylenediamhetetraacetic acid, an amino polycarboxylic acid. It is a tetraprotic acid and is represented as H4Y with four carboxyl groups and two nitrogen atoms acting as ligand sites. Ligands include ions such as Cl-, NO2-and CN- or neutral molecules like NH3 and H2O, which possess a lone pair of electrons that can be shared with a metal cation in coordinate covalent bonds.
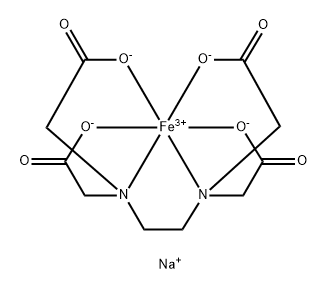
EDTA metal complexes, such as NaFeEDTA, MnEDTA, ZnEDTA and CuEDTA are used as fertilizers and foliar sprays.
Pharmaceutical Applications
The stability of the metal–ethylenediaminetetraacetic acid complex depends on the metal ion involved and also on the pH. The calcium chelate is relatively weak and will preferentially chelate heavy metals, such as iron, copper, and lead, with the release of calcium ions. For this reason, edetate calcium disodium is used therapeutically in cases of lead poisoning.
Biological Activity
Chelating agent; sequesters di- and trivalent metal ions.
Biochem/physiol Actions
Ethylenediaminetetraacetic acid (EDTA) is an anticoagulant, majorly used in preventing clotting of blood samples. EDTA may trigger aggregation of platelets resulting in pseudothrombocytopenia. EDTA along with tetracycline imparts serves as root conditioning agent and provides relief in gum infection. EDTA-tetracycline therapy decreases coronary artery calcium levels in atherosclerosis. Calcium Disodium EDTA administration is effective in slowing down the progression of chronic kidney disease.
Pharmacokinetics
Edetate calcium is a heavy metal chelating agent. The calcium in edetate calcium can be displaced by divalent or trivalent metals to form a stable water soluble complex that can be excreted in the urine. In theory, 1 g of edetate calcium can theoretically bind 620 mg of lead, but in reality only about 5 mg per gram is actually excreted into the urine in lead poisoned patients. In addition to chelating lead, edetate calcium also chelates and eliminates zinc from the body. Edetate calcium also binds cadmium, copper, iron and manganese, but to a much lesser extent than either lead or zinc. Edetate calcium is relatively ineffective for use in treating mercury, gold or arsenic poisoning.
Safety Profile
Poison by intraperitoneal route. Experimental teratogenic and reproductive effects. Mutation data reported. A general-purpose chelaung and complexing agent. When heated to decomposition it emits toxic fumes of NOx.
Safety
Edetic acid and edetates are widely used in topical, oral, and
parenteral pharmaceutical formulations. They are also extensively
used in cosmetics and food products.
Edetic acid is generally regarded as an essentially nontoxic and
nonirritant material, although it has been associated with doserelated
bronchoconstriction when used as a preservative in
nebulizer solutions. It has therefore been recommended that
nebulizer solutions for bronchodilation should not contain edetic
acid.
Edetates, particularly disodium edetate and edetate calcium
disodium, are used in a greater number and variety of pharmaceutical
formulations than the free acid.
Disodium edetate, trisodium edetate, and edetic acid readily
chelate calcium and can, in large doses, cause calcium depletion
(hypocalcemia) if used over an extended period or if administered
too rapidly by intravenous infusion. If used in preparations for the
mouth, they can also leach calcium from the teeth. In contrast,
edetate calcium disodium does not chelate calcium.
Edetate calcium disodium is nephrotoxic and should be used
with caution in patients with renal impairment.
The WHO has set an estimated acceptable daily intake for
disodium edetate in foodstuffs at up to 2.5 mg/kg body-weight.
LD50 (mouse, IP): 0.25 g/kg
LD50 (rat, IP): 0.397 g/kg
Potential Exposure
EDTA is a white, odorless, crystalline material or white powder
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
EDTA can be very persistent in water, including wastewatertreatment plants. EDTA is often found in the receiving waters of many industrial areas, thus being classified as one of the major organic pollutants discharged in waters. The available ecotoxicity data for EDTA indicate that these compounds are slow to degrade under typical environmental conditions but are not expected to bioconcentrate. EDTA compounds range from practically nontoxic to moderately toxic on an acute basis, depending on the salt. Algae and invertebrates are among the most sensitive species based on predictive modeling for acute and chronic endpoints for EDTA, depending on the compound. EDTA and its salts also do not appear to be very toxic for terrestrial wild mammals, and adverse effects from reasonably expected agricultural uses are not expected.
Metabolism
Almost none of the compound is metabolized.
Storage
Although edetic acid is fairly stable in the solid state, edetate salts
are more stable than the free acid, which decarboxylates if heated
above 150°C. Disodium edetate dihydrate loses water of crystallization
when heated to 120°C. Edetate calcium disodium is slightly
hygroscopic and should be protected from moisture.
Aqueous solutions of edetic acid or edetate salts may be sterilized
by autoclaving, and should be stored in an alkali-free container.
Edetic acid and edetates should be stored in well-closed
containers in a cool, dry place.
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
Dissolve EDTA in aqueous KOH or ammonium hydroxide, and precipitate it twice with dilute HCl or HNO3. Boil it twice with distilled water to remove mineral acid, then recrystallise it from water or dimethylformamide. Dry it at 110o. It also recrystallises from boiling 1N HCl; wash the crystals with distilled H2O and dry them in vacuo. [Ma & Ray Biochemistry 19 751 1980, Beilstein 4 IV 2449.]
Toxicity evaluation
The principal toxicity of EDTA relates to the metal chelate, especially in lead poisoning. Lead may be released from the chelate in the kidneys, and then the lead may affect the tubules and glomeruli of the kidneys.
Incompatibilities
Edetic acid and edetates are incompatible with strong oxidizing
agents, strong bases, and polyvalent metal ions such as copper,
nickel, and copper alloy.
Edetic acid and disodium edetate behave as weak acids,
displacing carbon dioxide from carbonates and reacting with
metals to form hydrogen.
Other incompatibilities include the inactivation of certain types
of insulin due to the chelation of zinc, and the chelation of trace
metals in total parenteral nutrition (TPN) solutions following the
addition of TPN additives stabilized with disodium edetate.
Calcium disodium edetate has also been reported to be incompatible
with amphotericin and with hydralazine hydrochloride in infusion
fluids.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral, otic, rectal, and topical preparations; submucosal injection preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Ethylenediaminetetraacetic acid
| Melting point: | 250 °C (dec.) (lit.) |
| Boiling point: | 434.18°C (rough estimate) |
| Density | 1.46 g/cm3 at 20 °C |
| vapor pressure | <0.013 hPa (20 °C) |
| refractive index | n |
| Flash point: | >400°C DIN 51758 |
| storage temp. | 2-8°C |
| solubility | 3 M NaOH: 100 mg/mL |
| form | crystalline |
| pka | pKa 2 (Uncertain);10.26 (Uncertain) |
| color | White to almost white |
| Odor | Odorless |
| PH | 2.5 (10g/l, H2O, 23℃)(slurry) |
| PH Range | 2.5 at 10 g/l at 23 °C |
| Water Solubility | 0.5 g/L (25 ºC) |
| λmax | λ: 280 nm Amax: ≤0.25 |
| Decomposition | 240 °C |
| Merck | 14,3517 |
| BRN | 1716295 |
| Stability: | Stable. Incompatible with copper, copper alloys, nickel, aluminium, strong oxidizing agents, strong bases |
| CAS DataBase Reference | 60-00-4(CAS DataBase Reference) |
| NIST Chemistry Reference | N,N'-1,2-Ethane diylbis-(N-(carboxymethyl)glycine)(60-00-4) |
| EPA Substance Registry System | Ethylenediaminetetraacetic acid (60-00-4) |
Safety information for Ethylenediaminetetraacetic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Ethylenediaminetetraacetic acid
Ethylenediaminetetraacetic acid manufacturer
JSK Chemicals
Remedy Labs
Bhimani Group
Techno Pharmchem
C.H. Chemicals
ASM Organics
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 60-00-4 98%View Details
60-00-4 98%View Details
60-00-4 -
 EDTA Acid 60-00-4 97%View Details
EDTA Acid 60-00-4 97%View Details
60-00-4 -
 Ethylenediaminetetraacetic acid CAS 60-00-4View Details
Ethylenediaminetetraacetic acid CAS 60-00-4View Details
60-00-4 -
 Ethylenediaminetetraacetic acid CAS 60-00-4View Details
Ethylenediaminetetraacetic acid CAS 60-00-4View Details
60-00-4 -
 Ethylenediaminetetraacetic acid CAS 60-00-4View Details
Ethylenediaminetetraacetic acid CAS 60-00-4View Details
60-00-4 -
 EDTA Free Acid extrapure AR CAS 60-00-4View Details
EDTA Free Acid extrapure AR CAS 60-00-4View Details
60-00-4 -
 EDTA Free Acid extrapure CAS 60-00-4View Details
EDTA Free Acid extrapure CAS 60-00-4View Details
60-00-4 -
 Ethylenediaminetetraacetic acid 99.00% CAS 60-00-4View Details
Ethylenediaminetetraacetic acid 99.00% CAS 60-00-4View Details
60-00-4
