Epinephrine Hydrochloride
- CAS NO.:55-31-2
- Empirical Formula: C9H14ClNO3
- Molecular Weight: 219.67
- MDL number: MFCD00801067
- EINECS: 200-230-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-30 09:03:32
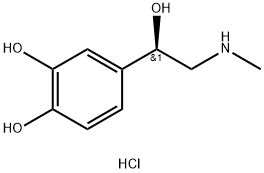
What is Epinephrine Hydrochloride?
General Description
Crystals. Used medically as a cardiostimulant.
Air & Water Reactions
Water soluble [Merck].
Reactivity Profile
L-Epinephrine hydrochloride is readily destroyed by alkalis and oxidizing agents .
Health Hazard
SYMPTOMS: Adverse reactions include restlessness; anxiety; headache; tremor; weakness; dizziness; pallor; respiratory difficulties; palpitation; nausea and vomiting.
Fire Hazard
Flash point data for L-Epinephrine hydrochloride are not available, but L-Epinephrine hydrochloride is probably combustible.
Mechanism of action
By stimulating vascular alpha-adrenergic receptors, epinephrine causes vasoconstriction, thereby increasing vascular resistance and blood pressure. When administered in the conjunctiva, Epinephrine Hydrochloride binds to alpha-adrenergic receptors in the iris sphincter muscle, resulting in vasoconstriction, a decrease in the production of aqueous humor, and a lowering of intraocular pressure. Through its beta1 receptor-stimulating actions, epinephrine increases the force and rate of myocardial contraction and relaxes bronchial smooth muscle, resulting in bronchodilation.
Pharmacology
Epinephrine Hydrochloride is the hydrochloride salt of the naturally occurring sympathomimetic amine with vasoconstricting, intraocular pressure-reducing, and bronchodilating activities.
Properties of Epinephrine Hydrochloride
| Melting point: | 157°C |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO > 10 mM |
| form | Powder |
| CAS DataBase Reference | 55-31-2(CAS DataBase Reference) |
| EPA Substance Registry System | Epinephrine hydrochloride (55-31-2) |
Safety information for Epinephrine Hydrochloride
Computed Descriptors for Epinephrine Hydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)



You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
