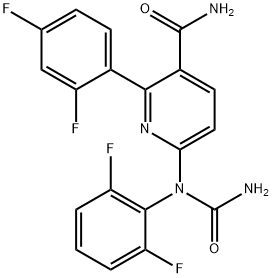
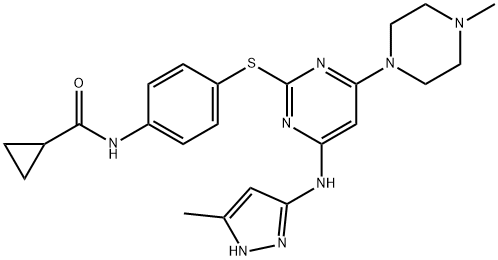
Elexacaftor
- CAS NO.:2216712-66-0
- Empirical Formula: C26H34F3N7O4S
- Molecular Weight: 597.65
- MDL number: MFCD32067875
- Update Date: 2026-02-02 18:10:39
What is Elexacaftor?
Absorption
The absolute oral bioavailability of elexacaftor is approximately 80%. The steady-state AUC0-24h and Cmax following once daily dosing with elexacaftor 200mg are 162 mcg?h/mL and 8.7 mcg/mL, respectively, and the median Tmax is 6 hours. The AUC of elexacaftor is increased 1.9-2.5-fold following a moderate-fat meal - for this reason, it is recommended to give TrikaftaTM with fat-containing food.
Toxicity
As elexacaftor is currently only available in the combination product TrikaftaTM (ivacaftor/tezacaftor/elexacaftor), data regarding overdose of elexacaftor on its own is unavailable. Treatment of TrikaftaTM overdose should consist of general supportive and symptomatic treatment, including monitoring of vital signs and close observation of the affected patient.
The Uses of Elexacaftor
Elexacaftor medication used in those that have cystic fibrosis with a f508del mutation.
Indications
Elexacaftor, in combination with ivacaftor and tezacaftor as the combination product TrikaftaTM, is indicated for the treatment of cystic fibrosis (CF) in patients 12 years of age and older who have at least one F508del mutation in the CTFR gene.
Background
Elexacaftor (previously VX-445) is a small molecule, next-generation corrector of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It received FDA approval in October 2019 in combination with tezacaftor and ivacaftor as the combination product TrikaftaTM. Elexacaftor is considered a next-generation CFTR corrector as it possesses both a different structure and mechanism as compared to first generation correctors like tezacaftor. While dual corrector/potentiator combination therapy has proven useful in the treatment of a subset of CF patients, their use is typically limited to patients who are homozygous for the F508del-CFTR gene. Elexacaftor, along with VX-659, was designed to fill the need for an efficacious CF therapy for patients who are heterozygous for F508del-CFTR and a gene that does not produce protein or produces proteins unresponsive to ivacaftor or tezacaftor. The triple combination product TrikaftaTM, manufactured by Vertex Pharmaceuticals, is the first product approved for the treatment of CF in individuals who are either homo- or heterozygous for the F508del-CFTR gene - this represents approximately 70-90% of all CF patients.
Pharmacokinetics
As a CFTR corrector, elexacaftor works to increase the amount of mature CFTR proteins present on the surface of cells. When used in combination with CFTR potentiators, which enhance the function of cell-surface CFTR proteins, drugs like elexacaftor help to improve a variety of multi-organ cystic fibrosis symptoms, including lung function, nutritional status, and overall quality of life. TrikaftaTM, the triple combination product containing elexacaftor, may cause elevations in liver transaminases. Liver function testing should be conducted prior to beginning Trikafta, every 3 months for the first year of treatment, and annually thereafter.
Metabolism
The metabolism of elexacaftor is extensive and primarily catalyzed via CYP3A4/5. Its main active metabolite, M23-ELX, carries a similar potency as the parent drug. The precise metabolic pathway of elexacaftor has not yet been elucidated in published research.
Properties of Elexacaftor
| Melting point: | 206 - 208°C |
| Density | 1.38±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 3.80±0.10(Predicted) |
| color | White to Off-White |
Safety information for Elexacaftor
Computed Descriptors for Elexacaftor
New Products
4-Iodo-3,5-dimethylbenzonitrile 2-fluoro-4-iodoaniline 2-(3-Methyl-1,2,4-oxadiazol-5-yl)acetic acid (as potassium salt) 6,6'-methylenebis(9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-2,3-dihydro-1H-carbazol-4(9H)-one) 3-Bromo-2-fluorobenzoic acid Quinuclidine-4-carbonitrile N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 1,4-bis(methylsulfonyl)butane 4-Cyano-N-Methacryloyl-3-Trifluoromethyl Aniline 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzeneRelated products of tetrahydrofuran
You may like
-
![6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
2115779-15-0 -
 4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
13745-17-0 -
 230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 -
 1941177-45-2 98%View Details
1941177-45-2 98%View Details
1941177-45-2 -
 Isopropyl amine Hydrochloride 99%View Details
Isopropyl amine Hydrochloride 99%View Details
15572-56-2 -
 19501-58-7 99%View Details
19501-58-7 99%View Details
19501-58-7 -
 Cyclopropane-1,1-dicarboxylic acid 99%View Details
Cyclopropane-1,1-dicarboxylic acid 99%View Details
598-10-7 -
 230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8