Elacestrant
- CAS NO.:722533-56-4
- Empirical Formula: C30H38N2O2
- Molecular Weight: 458.63
- MDL number: MFCD30532693
- EINECS: 258-984-3
- Update Date: 2026-01-20 19:14:41
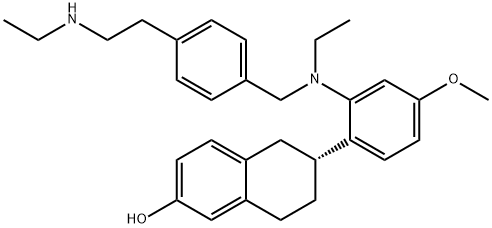
What is Elacestrant?
Absorption
With the recommended dosage of 345 mg once daily, elacestrant has a steady-state Cmax of 119 ng/mL and an AUC0-24h of 2440 ng?h/mL. The Cmax and AUC of elacestrant increase more than dose-proportional between 43 mg and 862 mg once daily (0.125 to 2.5 times the approved recommended dosage). By day 6, elacestrant reaches steady-state and has a 2-fold mean accumulation ratio based on AUC0-24h. The tmax of elacestrant goes from 1 to 4 hr, and its oral bioavailability is approximately 10%. Compared to a fasted state, the Cmax and AUC of elacestrant (345 mg) were 42% and 22% higher, respectively, when administered with a high-fat meal (800 to 1000 calories, 50% fat).
Toxicity
Toxicity information regarding elacestrant is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as dyslipidemia and gastrointestinal disorders. Symptomatic and supportive measures are recommended. The carcinogenicity of elacestrant has not been evaluated. Elacestrant did not show mutagenicity in the in vitro Ames assay and was negative for clastogenicity in in vitro chromosome aberration assays and the in vivo rat bone marrow micronucleus assay.
Fertility studies with elacestrant in animals have not been performed. Rats and cynomolgus monkeys presented adverse reactions in female reproductive organs including atrophy of the vagina, cervix, and uterus and follicular cysts in the ovary after receiving repeated doses of elacestrant. Male rats presented decreased cellularity of Leydig cells and degeneration/atrophy of the seminiferous epithelium in the testis.
Background
Elacestrant is a non-steroidal small molecule and an estrogen receptor (ER) antagonist. In January 2023, it was approved by the FDA for the treatment of ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. Elacestrant binds to estrogen receptor-alpha (ERα) and acts as a selective estrogen receptor degrader (SERD) thanks to its ability to block the transcriptional activity of the ER and promote its degradation. Other types of endocrine therapy, such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs), may lead to drug resistance over time; therefore, the use of a SERD represents a therapeutic approach for the treatment of endocrine-resistant breast cancers. Unlike fulvestrant, another FDA-approved SERD, elacestrant is orally bioavailable.
Indications
Elacestrant is indicated for the treatment of postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy.
Pharmacokinetics
The exposure-response relationships and pharmacodynamics time course of elacestrant have not been fully characterized. At the approved recommended dose, the use of elacestrant does not lead to QTc interval increases higher than 20 msec. Hypercholesterolemia and hypertriglyceridemia have occurred in patients taking elacestrant, and administering this drug to pregnant women may cause fetal harm. Unlike other selective estrogen receptor modulators and degraders, elacestrant is capable of crossing the blood-brain barrier.
Metabolism
Elacestrant is metabolized in the liver, mainly by CYP3A4 and, to a lesser extent, by CYP2A6 and CYP2C9.
Properties of Elacestrant
| Boiling point: | 609.5±55.0 °C(Predicted) |
| Density | 1.111±0.06 g/cm3(Predicted) |
| pka | 10.36±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Elacestrant
Computed Descriptors for Elacestrant
| InChIKey | SIFNOOUKXBRGGB-AREMUKBSSA-N |
| SMILES | C1=C2C(C[C@H](C3=CC=C(OC)C=C3N(CC)CC3=CC=C(CCNCC)C=C3)CC2)=CC=C1O |
Elacestrant manufacturer
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateYou may like
-
 722533-56-4 98+View Details
722533-56-4 98+View Details
722533-56-4 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
