DIBENZYL CARBONATE
- CAS NO.:3459-92-5
- Empirical Formula: C15H14O3
- Molecular Weight: 242.27
- MDL number: MFCD00014436
- EINECS: 222-401-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:22:34
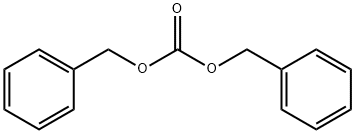
What is DIBENZYL CARBONATE?
Chemical properties
Colorless low melting solid
The Uses of DIBENZYL CARBONATE
Ionic liquids can effectively accelerate slow N-benzylation reactions utilizing dibenzyl carbonate as an alkylating reagent. Dibenzyl carbonate (DBzlC) has been used to benzylate phenylacetonitrile, benzyl phenylacetate and phenol. Selective N, N-dibenzylation of primary aliphatic amines was carried with dibenzyl carbonate in the presence of phosphonium salts.
Preparation
To a stirred solution of benzyl alcohol (0.216 g, 2.00 mol), tributylphosphine (0.303 g, 1.50 mmol), and CyTMG (0.394 g, 2.00 mmol) in DMF (2.00 mL), CO2 was added at room temperature. After 15 min, tetrabromomethane (0.663 g, 2.00 mmol) was added, the reaction vessel was sealed, and the contents were stirred for 2 h. Thereafter, the reaction mixture was diluted with ethyl acetate, washed successively with 0.5 m aqueous HCl and saturated aq. NaHCO3 solution, and dried over Na2SO4. Diphenylmetha- nol was added to the organic solution as an internal standard and the yield of dibenzyl carbonate was determined from the relative integrals of a methylene peak of the carbonate and a methine peak of diphenylmethanol in the 1H NMR spectrum of the ethyl acetate solution. Dibenzyl carbonate, prepared by a larger scale reaction of CO2 with benzyl alcohol (1.08 g, 10.0 mmol), was isolated by column chromatography (cyclohexane/ethyl acetate, 50:1) in 67.3% yield (0.816 g).
Synthesis Reference(s)
The Journal of Organic Chemistry, 60, p. 6205, 1995 DOI: 10.1021/jo00124a044
General Description
Dibenzyl carbonate (DBC) is commonly used as a benzylating agent. Dimethyl carbonate and excess of benzyl alcohol undergoes transesterification in the presence of CsF/α-Al2O3 (cesium fluoride/aluminum oxide) to form DBC. The formation of N,N-dibenzyl derivatives by the reaction of primary aliphatic amines with DBC in the presence of phosphonium salts has been investigated.
Properties of DIBENZYL CARBONATE
| Melting point: | 29-33 °C(lit.) |
| Boiling point: | 180-190 °C2 mm Hg(lit.) |
| Density | 1.1515 (rough estimate) |
| refractive index | 1.5485 |
| Flash point: | >230 °F |
| solubility | Sparingly Soluble (0.099 g/L) (25°C). |
| form | powder to lump |
| color | White to Almost white |
| Sensitive | Air Sensitive |
| BRN | 1882864 |
| CAS DataBase Reference | 3459-92-5(CAS DataBase Reference) |
| EPA Substance Registry System | Carbonic acid, bis(phenylmethyl) ester (3459-92-5) |
Safety information for DIBENZYL CARBONATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P321:Specific treatment (see … on this label). P501:Dispose of contents/container to..… |
Computed Descriptors for DIBENZYL CARBONATE
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 Dibenzyl Carbonate CAS 3459-92-5View Details
Dibenzyl Carbonate CAS 3459-92-5View Details
3459-92-5 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
