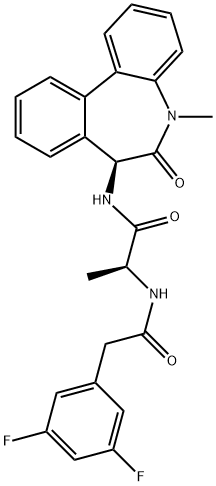
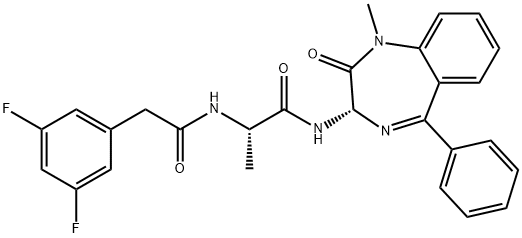
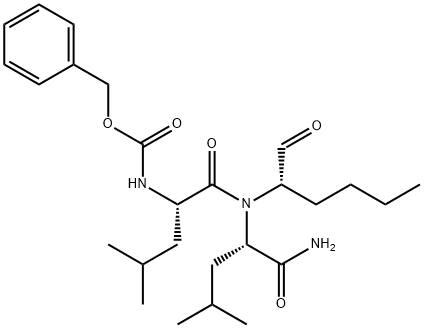
DIBENZAZEPINE
Synonym(s):DBZ;Dibenzazepine;GSI-XX;N-[(1S)-2-[[(7S)-6,7-Dihydro-5-methyl-6-oxo-5H-dibenz[b,d]azepin-7-yl]amino]-1-methyl-2-oxoethyl]-3,5-difluoro-benzeneacetamide;γ-Secretase inhibitor XX
- CAS NO.:209984-56-5
- Empirical Formula: C26H23F2N3O3
- Molecular Weight: 463.48
- MDL number: MFCD12828734
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 19:14:00
What is DIBENZAZEPINE?
Description
γ-
Chemical properties
White Solid
The Uses of DIBENZAZEPINE
DIBENZAZEPINE is a dipeptidic-secretase inhibitor and a antiAlzheimer agent.
The Uses of DIBENZAZEPINE
A dipeptidic γ-secretase inhibitor. AntiAlzheimer agent.
The Uses of DIBENZAZEPINE
γ-Secretase is a multi-subunit aspartyl protease that cleaves amyloid precursor protein (APP) and many other type 1 transmembrane proteins, including Notch, E-cadherin, and ErbB4. The proteolysis of APP by secretases produces beta amyloid (Aβ), a 39- to 42-amino acid peptide which forms the amyloid plaques that are characteristic of Alzheimer’s disease. DBZ is a dipeptidic inhibitor of γ-secretase that potently blocks the cleavage of Notch into its active signaling effector, Notch intracellular domain, in human T cell lymphoma (SupT1) cells (IC50 = 1.7 nM). Within 4 hours after a single 100 μM/kg dose, DBZ demonstrates anti-Alzheimer activity in an APP transgenic mouse model characterized by high levels of Aβ40 by reducing Aβ40 levels by 71%.
What are the applications of Application
Dibenzazepine (Deshydroxy LY 411575) is a dipeptidic (c)-secretase inhibitor
General Description
A cell-permeable, potent γ-secretase inhibitor that significantly lowers both brain and plasma Aβ40 levels by ~72% in Tg2576 mutant APP transgenic mouse model (100 μmol/kg, b.i.d). Induces a heterogeneous reorganization of the crypt structural-proliferative units in the intestinal tract and of the stem cell niche in the colon. Also potently inhibits Notch processing (IC50 = 1.7 nM in SupT1 cells) and induces conversion of proliferative crypt cells to post-mitotic goblet cells in both the C57BL/6 and ApcMin mouse models (10 μmol/kg, i.p).
Biochem/physiol Actions
Cell permeable: yes
Storage
-20°C
Properties of DIBENZAZEPINE
| Melting point: | 257-259?C |
| Boiling point: | 801.3±65.0 °C(Predicted) |
| Density | 1.35 |
| storage temp. | -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| pka | 12.76±0.20(Predicted) |
| form | White solid |
| color | white to beige |
Safety information for DIBENZAZEPINE
Computed Descriptors for DIBENZAZEPINE
| InChIKey | QSHGISMANBKLQL-OWJWWREXSA-N |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 Deshydroxy LY-411575 >95% CAS 209984-56-5View Details
Deshydroxy LY-411575 >95% CAS 209984-56-5View Details
209984-56-5 -
 γ-Secretase Inhibitor XX CAS 209984-56-5View Details
γ-Secretase Inhibitor XX CAS 209984-56-5View Details
209984-56-5 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
