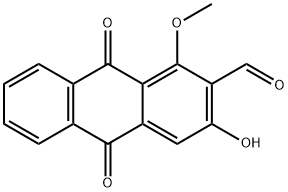
DAMNACANTHAL
Synonym(s):3-Hydroxy-1-methoxyanthraquinone-2-aldehyde;Damnacanthal - CAS 477-84-9 - Calbiochem
- CAS NO.:477-84-9
- Empirical Formula: C16H10O5
- Molecular Weight: 282.25
- MDL number: MFCD00674209
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:10
What is DAMNACANTHAL?
Description
Damnacanthal (477-84-9) is a potent inhibitor of p56lck?tyrosine kinase activity (IC50?= 17 nM for inhibition of autophosphorylation). Displays 7- to 20-fold selectivity over p59fyn?and p60src?tyrosine kinases and > 40-fold to 100-fold selectivity over other kinases.1?Mobilizes intracellular Ca2+?in dermal fibroblasts.2?Inhibits LIM-kinase, impairing cell migration and invasion.3?A potent inhibitor of angiogenesis.4?Inhibits mast cell activation.5
Description
Damnacanthal, an anthraquinone derivative isolated from the noni plant (Morinda citrifolia), has been touted as a phytochemical whose beneficial effects include HIV protein inhibition. But the lack of scientific proof for these claims caused the FDA to issue warning letters to its purveyors during 2002–2006.
The Uses of DAMNACANTHAL
Damnacanthal-induced anti-inflammation is associated with inhibition of NF-κB activity. Damnacanthal is used in the treatment of inflammatory-related diseases.
What are the applications of Application
Damnacanthal is an immunomodulator and potent inhibitor of p56lck tyrosine kinase
Definition
ChEBI: 3-hydroxy-1-methoxy-9,10-dioxo-2-anthracenecarboxaldehyde is a monohydroxyanthraquinone and an aldehyde.
References
1) Faltynek?et al.?(1995),?Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity; Biochemistry,?34?12404 2) Aoki?et al.?(2000),?Mechanism of damnacanthal-induced ;Ca(2+)](i) elevation in human dermal fibroblasts; Eur. J. Pharmacol.,?387?119 3) Ohashi?et al.?(2014),?Damnacanthal, an effective inhibitor of LIM-kinase, inhibits cell migration and invasion; Mol. Biol. Cell,?25?828 4) Garcia-Vilas?et al.?(2017),?The noni anthraquinone damnacanthal is a multi-kinase inhibitor with potent anti-angiogenic effects; Cancer Letters,?385?1 5) Garcia-Vilas?et al.?(2015),?Damnacanthal inhibits IgE receptor-mediated activation of mast cells; Mol. Immunol.,?65?86
Properties of DAMNACANTHAL
| Melting point: | 218~219℃ |
| Boiling point: | 531.9±50.0 °C(Predicted) |
| Density | 1.461±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 7 mg/ml) |
| form | Pale orange solid. |
| pka | 5.26±0.20(Predicted) |
| color | Yellow |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
Safety information for DAMNACANTHAL
Computed Descriptors for DAMNACANTHAL
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran

You may like
-
 Damnacanthal CAS 477-84-9View Details
Damnacanthal CAS 477-84-9View Details
477-84-9 -
 DamnacanthalView Details
DamnacanthalView Details
477-84-9 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
