Cyclohexane
Synonym(s):Cyclohexane;Cyclohexane in DMSO;Cyclohexane solution;Hexahydrobenzene;Hexahydrobenzene, Cyclohexane
- CAS NO.:110-82-7
- Empirical Formula: C6H12
- Molecular Weight: 84.16
- MDL number: MFCD00003814
- EINECS: 203-806-2
- Update Date: 2026-01-13 11:19:40
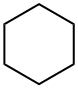
What is Cyclohexane?
Description
Cyclohexane (C6H12) is an alicyclic hydrocarbon with six carbon atoms in its ring. It is primarily used as a raw material for the manufacture of nylon. It is also used in machinery and automotive lubricants, greases, and hydraulic fluids, fuels, antifreeze products, coatings, non-metallic surface treatments, adhesives and sealants, and inks and toners. Household uses include machine washes/detergents, car care products, paints and coatings/adhesives, fragrances and air fresheners, refrigerator coolants, and oil-based electric heaters.
Physical properties
Cyclohexane is a colorless liquid with a mild sweet odor. It is flammable and insoluble in water, but soluble in organic solvents such as alcohol, ether, and acetone. Its density is lower than that of water. The experimental odor threshold concentration is 2,700 mg/m3 (784 ppmv) or 2.7 ppbv.
The Uses of Cyclohexane
Cyclohexane is widely used as a solvent for paints and resins, a paint and varnish remover, and in the manufacture of adipic acid, benzene, cyclohexyl chloride, nitrocyclohexane, cyclohexanol, and cyclohexanone. It is also used in the extraction of essential oils; in the manufacture of solid fuel for camping stoves; in the formulation of fungicides (where it has a mild fungicidal effect); and in the industrial recrystallization of steroids.
The Uses of Cyclohexane

A suspension of NaH (60% dispersion on mineral oil, 413 mg, 10.32 mmol) in dry DMF (12 mL) at 0 C was treated dropwise with the thiol (B) (1.21 mL, 10.32 mmol). After the addition the mixture was stirred at RT for 15 min. The aryl chloride (A) (1.00 g, 3.44 mmol) was added and the reaction mixture was stirred at RT for 18 h, 80 C for 8 h, then allowed to cool overnight. The mixture was diluted with EtOAc, washed with H2O, brine, dried (Na2SO4), and concentrated in vacuo. The resulting material was purified by silica gel column chromatography (30-50% EtOAc/cyclohexane) to provide the product. [659 mg, 51%]
Definition
ChEBI: Cyclohexane is an alicyclic hydrocarbon comprising a ring of six carbon atoms; the cyclic form of hexane, used as a raw material in the manufacture of nylon. It has a role as a non-polar solvent. It is a cycloalkane and a volatile organic compound.
Production Methods
Cyclohexane is fractionated from crude oil and may be released wherever petroleum products are refined, stored, and used. Another large source of general release is in exhaust gases from motor vehicles. It is prepared synthetically from benzene, by hydrocracking of cyclopentane, or from toluene by simultaneous dealkylation and double bond hydrogenation.
General Description
Cyclohexane is a colorless liquid cycloalkane with the molecular formula C6H12; a reaction end value of 0.78; a melting point of 6.5°C; a boiling point of 81°C; and a petroleum-like odor. It is found in petroleum and can be prepared by reacting benzene and hydrogen under pressure over a Raney nickel catalyst heated to 150°C, or by reduction with cyclohexanone. It is used as a solvent and paint stripper and can be oxidized to adipic acid (adipic acid) with hot concentrated nitric acid. The cyclohexane ring is not planar and can adopt boat and chair configurations; in chemical formulas, it is represented by a hexagon.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Liquid nitrogen dioxide was fed into a nitration column containing hot Cyclohexane, due to an error. An explosion resulted [MCA Case History 128(1962)] Incompatible with strong oxidizers.
Health Hazard
Cyclohexane is an acute toxicant of loworder. It is an irritant to the eyes and respiratorysystem. Exposure to a 1–2% concentrationin air caused lethargy, drowsiness,and narcosis in test animals. The lethal concentrationfor a 1-hour exposure in mice isestimated at around 30,000 ppm. Ingestionof cyclohexane exhibited low toxic effects intest species. The LD50 values in the literature show a wide variation. It may be detectedfrom its odor at 300 ppm concentration.
Flammability and Explosibility
Highly flammable
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by intravenous route, Moderately toxic by ingestion. A systemic irritant by inhalation and ingestion. A skin irritant. Mutation data reported. Flammable liquid. Dangerous fire hazard when exposed to heat or flame; can react with oxidming materials. Moderate explosion hazard in the form of vapor when exposed to flame. When mixed hot with liquid dinitrogen tetraoxide an explosion can result. To fight fire, use foam, CO2, dry chemical, spray, fog. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
Cyclohexane is used as a chemical intermediate; as a solvent for fats, oils, waxes, resins, cer- tain synthetic rubbers; and as an extractant of essential oils in the perfume industry.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention. Donot induce vomiting
Carcinogenicity
No tumor-promoting activity was observed in mice receiving one 60 mL application of 0.3% DMBA in benzene followed by a 60 mL application of cyclohexane twice weekly for 50 weeks.
Source
Schauer et al. (1999) reported cyclohexane in a diesel-powered medium-duty truck
exhaust at an emission rate of 210 μg/km.
California Phase II reformulated gasoline contained cyclohexane at a concentration of 8,900
mg/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 1.44 and 238.0 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Biological. Microbial degradation products reported include cyclohexanol (Dugan, 1972;
Verschueren, 1983), 1-oxa-2-oxocycloheptane, 6-hydroxyheptanoate, 6-oxohexanoate, adipic acid,
acetyl-CoA, succinyl-CoA (quoted, Verschueren, 1983), and cyclohexanone (Dugan, 1972; Keck
et al., 1989).
Photolytic. The following rate constants were reported for the reaction of cyclohexane and OH
radicals in the atmosphere: 5.38 x 10-12 cm3/molecule?sec at 295 K (Greiner, 1970); 6.7 x 10-12
cm3/molecule?sec at 300 K (Darnall et al., 1978); 6.69 x 10-12 cm3/molecule?sec at 298 (DeMore
and Bayes, 1999); 7.0 x 10-12 cm3/molecule?sec (Atkinson et al., 1979); 7.49 x 10-12
cm3/molecule?sec (Atkinson, 1990). A photooxidation reaction rate constant of 1.35 x 10-16
cm3/molecule?sec was reported for the reaction of cyclohexane with NO3 in the atmosphere
(Atkinson, 1991).
Chemical/Physical. The gas-phase reaction of cyclohexane with OH radicals in the presence of
nitric oxide yielded cyclohexanone and cyclohexyl nitrate as the major products (Aschmann et al.,
1997).
Cyclohexane will not hydrolyze because it has no hydrolyzable functional group.
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with cyclohexane you should be trained onits proper handling and storage. Before entering confinedspace where cyclohexane may be present, check to makesure that an explosive concentration does not exist.Cyclohexane must be stored to avoid contact with oxidizers(such as perchlorates, peroxides, permanganates, chlorates,and nitrates), since violent reactions occur. Store in tightlyclosed containers in a cool well-ventilated area away fromheat. Metal containers involving the transfer of this chemical should be grounded and bonded. Where possible, automatically pump liquid from drums or other storagecontainers to process containers. Drums must be equippedwith self-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Sources of ignition, such as smoking and open flames,are prohibited where this chemical is used, handled, orstored in a manner that could create a potential fire orexplosion hazard. Wherever cyclohexane is used, handled,manufactured, or stored, use explosion-proof electricalequipment and fittings.
Shipping
UN1145 Cyclohexane, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
It is best to purify it by washing with conc H2SO4 until the washings are colourless, followed by water, aqueous Na2CO3 or 5% NaOH, and again water until neutral. It is then dried with P2O5, Linde type 4A molecular sieves, CaCl2, or MgSO4 then Na and distilled. Cyclohexane has been refluxed with, and distilled from Na, CaH2, LiAlH4 (which also removes peroxides), sodium/potassium alloy, or P2O5. Traces of *benzene can be removed by passage through a column of silica gel that has been freshly heated: this gives material suitable for ultraviolet and infrared spectroscopy. If there is much *benzene in the cyclohexane, most of it can be removed by a preliminary treatment with nitrating acid (a cold mixture of 30mL conc HNO3 and 70mL of conc H2SO4) which converts *benzene into nitrobenzene. The impure cyclohexane and the nitrating acid are placed in an ice bath and stirred vigorously for 15minutes, after which the mixture is allowed to warm to 25o during 1hour. The cyclohexane is decanted, washed several times with 25% NaOH, then water, dried with CaCl2, and distilled from sodium. Carbonyl-containing impurities can be removed as described for chloroform. Other purification procedures include passage through columns of activated alumina and repeated crystallisation by partial freezing. Small quantities may be purified by chromatography on a Dowex 710-Chromosorb W gas-liquid chromatographic column. Flammable liquid. [Sabatier Ind Eng Chem 18 1005 1926, Schefland & Jacobs The Handbook of Organic Solvents (Van Nostrand) p592 1953, Beilstein 5 IV 27.] Rapid purification: Distil, discarding the forerun. Stand distillate over Grade I alumina (5% w/v) or 4A molecular sieves.
Toxicity evaluation
If released to air, cyclohexane will exist solely as a vapor in the
ambient atmosphere, and will be degraded in the atmosphere
by reaction with photochemically produced hydroxyl radicals,
though direct photolysis is not expected due to the lack of
absorption in the environmental spectrum. Volatilization from
water surfaces is expected to be an important fate for this
compound, and half-lives in a model river and lake are expected
to be 3 h and ~3.5 days respectively. Adsorption to
suspended solids and sediments is also expected, though
hydrolysis in the environment is unlikely due to the lack of
hydrolyzable functional groups.
The potential for bioconcentration and bioaccumulation of
cyclohexane in aquatic organisms is moderate. It is highly
resistant to biodegradation and is catabolized chiefly by
cooxidation.
Incompatibilities
May form explosive mixture with air. Contact with oxidizers, nitrogen dioxide, and oxygen can cause fire and explosion hazard. Can explode in heat when mixed with dinitrogen tetraoxide liquid.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Cyclohexane
| Melting point: | 4-7 °C (lit.) |
| Boiling point: | 80.7 °C (lit.) |
| Density | 0.779 g/mL at 25 °C (lit.) |
| vapor density | 2.9 (vs air) |
| vapor pressure | 168.8 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | -1 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | ethanol: miscible(lit.) |
| appearance | Colorless liquid |
| form | Liquid |
| color | colorless |
| Odor | resembling benzene; mild, sweet, resembling chloroform. |
| Relative polarity | 0.006 |
| explosive limit | 1.2-8.3%(V) |
| Odor Threshold | 2.5ppm |
| Water Solubility | PRACTICALLY INSOLUBLE |
| FreezingPoint | 6.554℃ |
| λmax | λ: 210 nm Amax: ≤1.00 λ: 220 nm Amax: ≤0.50 λ: 230 nm Amax: ≤0.20 λ: 235 nm Amax: ≤0.10 λ: 240 nm Amax: ≤0.08 λ: 250 nm Amax: ≤0.03 λ: 255 nm Amax: ≤0.01 |
| Merck | 14,2723 |
| BRN | 1900225 |
| Henry's Law Constant | 1.03, 1.26, 1.40, 1.77, and 2.23 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al.,
1988)
0.54, 0.69, 0.82, 1.43, and 1.79 at 2.0, 6.0, 10.0, 18.0, and 25.0 °C, respectively (Dewulf et
al.,1999) |
| Exposure limits | TLV-TWA 300 ppm (~1050 mg/m3)
(ACGIH, OSHA, and NIOSH); IDLH
10,000 ppm (NIOSH). |
| Dielectric constant | 2.0(20℃) |
| Stability: | Volatile |
| CAS DataBase Reference | 110-82-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexane(110-82-7) |
| EPA Substance Registry System | Cyclohexane (110-82-7) |
Safety information for Cyclohexane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Cyclohexane
| InChIKey | XDTMQSROBMDMFD-UHFFFAOYSA-N |
Cyclohexane manufacturer
JSK Chemicals
VJ CHEMTRADE PVT LTD
Crystal Chemitrade
Veera Chemical
True Chem Enterprise
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Cyclohexane 99%View Details
Cyclohexane 99%View Details -
 Cyclohexane 98%View Details
Cyclohexane 98%View Details -
 Cyclo Hexane 99%View Details
Cyclo Hexane 99%View Details -
 Cyclohexane 99%View Details
Cyclohexane 99%View Details -
 Cyclohexane, For ACS analysis CAS 110-82-7View Details
Cyclohexane, For ACS analysis CAS 110-82-7View Details
110-82-7 -
 Cyclohexane, For ACS analysis CAS 110-82-7View Details
Cyclohexane, For ACS analysis CAS 110-82-7View Details
110-82-7 -
 Cyclohexane, For ACS analysis CAS 110-82-7View Details
Cyclohexane, For ACS analysis CAS 110-82-7View Details
110-82-7 -
 Cyclohexane CASView Details
Cyclohexane CASView Details
