Creosote
Synonym(s):Beechwood creosote
- CAS NO.:8021-39-4
- Empirical Formula: C7H8O2
- Molecular Weight: 124.13722
- MDL number: MFCD00146582
- EINECS: 232-419-1
- Update Date: 2025-12-10 11:56:18
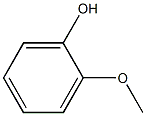
What is Creosote?
Chemical properties
Yellow to dark brown, oily liquid with a distinctive pungent odor
Occurrence
Fagus species.
The Uses of Creosote
Coal tar creosote has been used as a wood preservative pesticide in the United States since the late 1890s. This accounts for more than 97% of coal tar creosote production.Other uses include animal and bird repellent, insecticide, animal dip, fungicide, and pharmaceutical applications. Beechwood creosote has, in the past, been used for medicinal purposes.
The Uses of Creosote
Wood creosote has been used as a disinfectant, a laxative, and a cough treatment, but has since been replaced by better medicines. Coal tar products are used in medicines to treat skin diseases such as psoriasis, and also as animal and bird repellents, insecticides, animal dips, and fungicides. Coal tar creosote is the most widely used wood preservative in the United States. Coal tar, coal tar pitch, and coal tar pitch volatiles are used for roofing, aluminum smelting, and coking.
What are the applications of Application
Creosote is a biochemical from beechwood tar often used as a preservative and antiseptic
Definition
1. (wood creosote) An almostcolourless liquid mixture ofphenols obtained by distilling tar obtainedby the destructive distillationof wood. It is used medically as anantiseptic and expectorant. 2. (coaltarcreosote) A dark liquid mixture ofphenols and cresols obtained by distillingcoal tar. It is used for preservingtimber.
Preparation
Starting materials are the heavy oils from wood tar; the material first is treated with NaOH to precipitate all saponifiable oils and subsequently with H2SO4; the raw creosote finally is purified by repeated fractional distillation
Production Methods
Wood creosote is obtained from wood tars, from beech and the resin from leaves of the creosote bush, and by distillation and is composed mainly of phenols, xylenols, guaiacol, and creosol. Coal tar creosote is produced by high-temperature carbonization and distillation of bituminous coal. Coal tar creosote contains liquid and solid aromatic hydrocarbons, tar acids, and tar base.At least 75%of the coal tar creosote mixture is polyaromatic hydrocarbons. Purification of the crude preparation is accomplished by distillation and extraction from suitable oils.
General Description
Colorless to yellowish oily liquid with a smoky odor and caustic burning taste.
Air & Water Reactions
May be sensitive to exposure to air. Slightly soluble in water.
Reactivity Profile
Phenols, such as CREOSOTE, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. CREOSOTE is incompatible with acacia, albumin, oxidizers and cupric, ferric, gold and silver salts.
Safety Profile
Confirmed carcinogen. When heated to decomposition it emits acrid smoke and irritating fumes.There is a fire hazard, CREOSOTE is combustible.
Carcinogenicity
The carcinogenicity of creosote oils has been studied quite thoroughly using mice. Studies indicate that coal tar creosote and several of its fractions can be carcinogenic when applied to the skin of mice and rabbits. Dermally applied coal tar creosote can also act as a tumor-initiating agent when applied prior to croton oil treatment.
Hazard
In aquatic sediments, several reactions can transform the chemicals released by the creosote preservatives into more dangerous chemicals. Most creosote preservative compounds have hazards associated with them before they are transformed. Cresol (m-, p-, and o-), phenol, guaiacol, and xylenol (1,3,4- and 1,3,5-) all are acute aquatic hazards prior to going through chemical reactions with the sediments. Alkylation reactions allows for the compounds to transition into more toxic compounds with the addition of R-groups to the major compounds found in creosote preservatives. Compounds formed through alkylation include: 3,4-dimethylphenol, 2,3-dimethylphenol, and 2,5-dimethylphenol, which are all listed as acute environmental hazards.
Properties of Creosote
| Boiling point: | 200-220 °C(lit.) |
| Density | 1.09 g/mL at 20 °C |
| Flash point: | 74 °C |
| color | Yellowish, greasy |
| Odor | liquid with smokey odor |
| Merck | 13,2601 |
| EPA Substance Registry System | Wood creosote (8021-39-4) |
Safety information for Creosote
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H332:Acute toxicity,inhalation H341:Germ cell mutagenicity H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Creosote
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran








You may like
-
 8001-58-9 / 8021-39-4 Creosote 98%View Details
8001-58-9 / 8021-39-4 Creosote 98%View Details
8001-58-9 / 8021-39-4 -
 8001-58-9 / 8021-39-4 Creosote 98%View Details
8001-58-9 / 8021-39-4 Creosote 98%View Details
8001-58-9 / 8021-39-4 -
 Creosote (beachwood) CAS 8021-39-4View Details
Creosote (beachwood) CAS 8021-39-4View Details
8021-39-4 -
 Creosote from beechwood tar CAS 8021-39-4View Details
Creosote from beechwood tar CAS 8021-39-4View Details
8021-39-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
