Conivaptan hydrochloride
- CAS NO.:168626-94-6
- Empirical Formula: C32H26N4O2.HCl
- Molecular Weight: 535.04
- MDL number: MFCD00945712
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-23 16:19:27
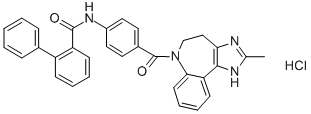
What is Conivaptan hydrochloride?
Description
Arginine vasopressin is intimately involved in volume homeostasis, and elevated levels of arginine vasopressin are responsible for the pathogenesis and progression of diseases with an imbalance of sodium and water, particularly congestive heart failure. To restore homeostasis, antagonism of vasopressin receptors is a practical solution. As such, conivaptan has been developed and launched as a dual V1a and V2 vasopressin receptor antagonist. As a competitive, reversible inhibitor of both subtypes, conivaptan can modulate systemic vascular resistance through the V1a receptor (Ki ? 0.48 nM) distributed in vascular smooth muscle cells, cardiomyocytes, hepatocytes, and platelets and blocks the renal V2 receptor (Ki ? 3.04 nM) resulting in enhanced diuresis, thereby increasing serum sodium concentration and reducing total body volume. Currently, the drug is approved for the management of refractory hyponatremia and potentially lifethreatening sodium and water imbalance, but it has shown promise as a potential treatment option for other diseases, such as congestive heart failure, syndrome of antidiuretic hormone, diabetes insipidus, and liver cirrhosis.
Description
Conivaptan is an antagonist of the arginine vasopressin (AVP) receptors V1A and V2 (Kis = 0.48 and 3.04 nM for rat liver V1A and kidney V2, respectively). It also competitively inhibits oxytocin binding to rat uterine oxytocin receptors (Ki = 44 nM) but has no effect on AVP binding to anterior pituitary V1B receptors at concentrations up to 100 μM in a radioligand binding assay. Conivaptan suppresses AVP-induced increases in intracellular calcium in vascular smooth muscle cells (VSMCs) in vitro and the pressor response in pithed rats. Conivaptan (0.01-0.3 mg/kg, i.v.) increases urine output and decreases urine osmolality in dehydrated conscious rats in a dose-dependent manner. It also reduces brain edema and blood-brain barrier disruption in a mouse experimental stroke model.
Chemical properties
White to Off-White Solid
Originator
Yamanouchi (Japan)
The Uses of Conivaptan hydrochloride
Conivaptan is a non-peptide inhibitor of antidiuretic hormone (vasopressin receptor antagonist).
The Uses of Conivaptan hydrochloride
Used in treatment of congestive heart failure.
What are the applications of Application
Conivaptan hydrochloride is a non-peptide inhibitor of the antidiuretic hormone
Definition
ChEBI: The hydrochloride salt of conivaptan. It is an antagonist for two of the three types of arginine vasopressin (AVP) receptors, V1a and V2, and is used for the treatment of hyponatraemia (low blood sodium levels) cau ed by syndrome of inappropriate antidiuretic hormone (SIADH).
brand name
Vaprisol (Astellas).
Storage
Desiccate at RT
Properties of Conivaptan hydrochloride
| Melting point: | >250° |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
Safety information for Conivaptan hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. |
Computed Descriptors for Conivaptan hydrochloride
Conivaptan hydrochloride manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Conivaptan hydrochloride 98% CAS 168626-94-6View Details
Conivaptan hydrochloride 98% CAS 168626-94-6View Details
168626-94-6 -
 Conivaptan hydrochloride 98% (HPLC) CAS 168626-94-6View Details
Conivaptan hydrochloride 98% (HPLC) CAS 168626-94-6View Details
168626-94-6 -
 Conivaptan hydrochloride CAS 168626-94-6View Details
Conivaptan hydrochloride CAS 168626-94-6View Details
168626-94-6 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
