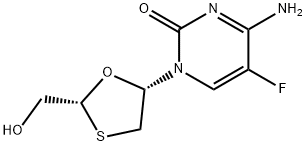
Clevudine
- CAS NO.:163252-36-6
- Empirical Formula: C10H13FN2O5
- Molecular Weight: 260.22
- MDL number: MFCD00935785
- EINECS: 200-001-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-23 16:19:27
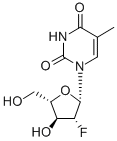
What is Clevudine?
Description
Clevudine is a fluorinated b-L-nucleoside analog launched for the
oral treatment of chronic HBV infection. It is the fifth nucleoside or nucleotide
analog to be marketed for this indication. The previous drugs from this class
include lamivudine, adefovir, entecavir, and telbivudine. In HBV-expressing
human hepatoma cell line 2.2.15, clevudine inhibits HBV DNA synthesis with
an EC50 of 0.1 μM, and does not show cytotoxicity up to 200 μM. It is
phosphorylated by cellular kinases to the active triphosphate derivative,
which subsequently inhibits HBV DNA polymerase and HBV replication.
466 Shridhar Hegde and Michelle Schmidt
Clevudine-5′-triphosphate has an intracellular half-life of 16.5 h. Interestingly, it
is a non-competitive inhibitor of viral polymerase, and inhibits HBV replication
without being incorporated into the DNA. The pharmacokinetic profile of clevudine was linear with a plasma half-life of approximately 60 h. Clevudine was undetectable in plasma after 4 weeks following the cessation of dosing.The most common adverse events reported with clevudine treatment include infection, asthenia, dyspepsia, abdominal pain, headache, and diarrhea.
Clevudine is chemically derived from L-ribose by first incorporating acyl protective groups to produce 1-O-acetyl-2,3,5-tri-O-benzoyl-b-L-ribofuranose intermediate, which is then converted to 1,3,5-tri-O-benzoyl-a-L-ribofuranose in two steps by treating To Market, To Market 2007 467 with hydrogen chloride and subsequent hydrolysis and acyl migration. The remaining steps leading to clevudine include conversion of the C2-hydroxy group to C2-fluoro group with triethylamine trihydrofluoride, formation of the corresponding ribofuranosyl bromide intermediate with hydrogen bromide and acetic acid, condensation with silylated thymine, and removal of benzoyl protective groups with methanolic ammonia.
Chemical properties
White Solid
Originator
University of Georgia/ Yale University (US)
The Uses of Clevudine
Antiviral drug, used for the treatment of Hepatitis B.
Definition
ChEBI: Clevudine is a pyrimidine 2'-deoxyribonucleoside.
brand name
Levovir
Synthesis
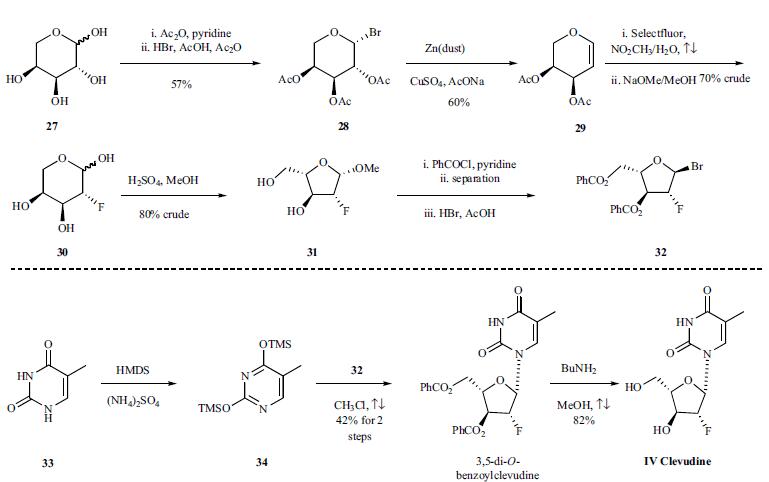
The synthesis is depicted in the scheme. L-Arabinose (27) was treated with acetic anhydride and pyridine at room temperature for four hours to give acetylated arabinose, which was then brominated using 30% HBr in AcOH/Ac2O at room temperature for 36 hours to afford bromo-sugar 28 as a white solid in 57% yield after crystallization in ethyl ether. Bromo-sugar 28 was then treated with Zn dust, CuSO4 and NaOAc in AcOH/H2O, followed by chromatographic separation to give L-arabinal 29 in 60% yield. L-arabinal 29 was converted to the fluoro derivative in 70% crude yield by reaction with Selectfluor® (FTEDA- BF4) in refluxing nitromethane/H2O, and the resulting fluoroalcohol was deacetylated with NaOMe in MeOH to give compound 30 in 100% crude yield. Compound 30 was then treated with H2SO4 in refluxing MeOH to afford methyl furanoside 31 in 80% crude yield. Furanoside 31 was benzoylated with benzoyl chloride in pyridine to give a mixture of isomers, from which the |á-anomer was isolated by chromatography and then brominated with 30% HBr/AcOH in CH2Cl2 to provide the crude bromo-sugar 32 which was dissolved in chloroform and used without further purification in the next step. Compound 34 was obtained by treatment of thymine (33) with HMDS and ammonium sulfate in refluxing chloroform for 16 hours. The sugar 32 was condensed with silylated pyrimidine derivative 34 in refluxing chloroform to afford 3,5-di-O-benzoylclevudine in 42% yield after recrystallization from ethanol. The benzoyl groups were removed upon treatment with n-butylamine in refluxing methanol to give clevudine (IV) in 82% yield.
Properties of Clevudine
| Melting point: | 184-185° |
| alpha | D25 -111.77° (c = 0.23 in methanol) |
| Density | 1.55±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 9.55±0.10(Predicted) |
| form | Solid |
| color | White |
| CAS DataBase Reference | 163252-36-6 |
Safety information for Clevudine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Clevudine
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Clevudine 95% CAS 163252-36-6View Details
Clevudine 95% CAS 163252-36-6View Details
163252-36-6 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
