CAMPESTEROL
Synonym(s):24α-Methyl-5-cholesten-3β-ol;24(R)-Ergost-5-en-3β-ol;campest-5-en-3β-ol
- CAS NO.:474-62-4
- Empirical Formula: C28H48O
- Molecular Weight: 400.69
- MDL number: MFCD00010475
- EINECS: 207-484-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:27:10
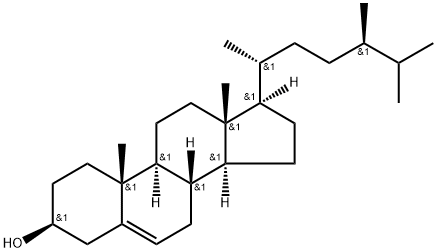
What is CAMPESTEROL?
Description
24α-methyl Cholesterol is a phytosterol found in vegetables, fruits, nuts, and seeds that competitively inhibits the absorption of intestinal cholesterol and decreases the transcription of genes important for cholesterol metabolism. The cholesterol lowering effects of phytosterols, such as 24α-methyl cholesterol, have been reported to be beneficial for lowering the incidence of atherosclerotic plaques. 24α-methyl Cholesterol can also act as an agonist at liver X receptors (LXR) and, through activation of LXR signaling, has been shown to suppress the proliferation of prostate and breast cancer cells.
Chemical properties
White Solid
The Uses of CAMPESTEROL
Campesterol may be used as a plant sterol to test its effect on the transcriptional activation of liver X receptor α (LXRA) in breast cancer cells. It may also be used as a sterol standard for calibration curve generation in liquid chromatography multiple reaction monitoring (LC-MRM) analysis.
The Uses of CAMPESTEROL
A phytosterol, which may inhibit the intestinal absorption of cholesterol.
What are the applications of Application
Campesterol is a plant sterol used for research purposes
Definition
ChEBI: Campesterol is a member of phytosterols, a 3beta-sterol, a 3beta-hydroxy-Delta(5)-steroid and a C28-steroid. It has a role as a mouse metabolite. It derives from a hydride of a campestane.
General Description
Campesterol is a plant sterol, which is an analog of cholesterol and a brassinosteroid (BR) precursor. The enzyme diminute/dwarf1 mediates the synthesis of campesterol from 24-methylenecholesterol. Campesterol possesses methyl group at C-24 position of the side chain in the cholesterol structure.
Biochem/physiol Actions
Campesterol is a phytosterol, primarily found in nuts, fruits, legumes and seeds. Though an analogue of cholesterol, it is poorly absorbed in humans and competitively inhibits the absorption of cholesterol. Campesterol decreases the transcription of genes involved in cholesterol metabolism in hepatocytes and enterocytes and has positive impact in treatment of cardiovascular disease.
Purification Methods
Campesterol is recrystallised twice from hexane and once from Me2CO. The benzoyl derivative has m 158-160o [] D 23 -8.6o (CHCl3), and the acetyl derivative has m 137138o (EtOH) and [] D 23 -35.1o (c 2.9, CHCl3) [Fernholz & MacPhillamy J Am Chem Soc 6 3 1155 1941]. [Beilstein 6 III 2680.]
Properties of CAMPESTEROL
| Melting point: | 156-160 °C |
| Boiling point: | 463.29°C (rough estimate) |
| alpha | D23 -33° (22.5 mg in 5 ml chloroform) |
| Density | 0.98±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5100 (estimate) |
| storage temp. | -20°C |
| solubility | Chloroform (Sparingly), Methanol (Slightly, Sonicated) |
| form | crystalline |
| pka | 15.03±0.70(Predicted) |
| color | white |
| Merck | 13,1736 |
| BRN | 3216975 |
Safety information for CAMPESTEROL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CAMPESTEROL
| InChIKey | SGNBVLSWZMBQTH-ZRUUVFCLSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran





You may like
-
 campesterol CAS 474-62-4View Details
campesterol CAS 474-62-4View Details
474-62-4 -
 Campesterol CAS 474-62-4View Details
Campesterol CAS 474-62-4View Details
474-62-4 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
